UW4 glycogen storage diseases Flashcards
question

D.

This patient most likely has a herpes simplex virus (HSV) infection. HSV classically presents with multiple, painful genital ulcers with a characteristic erythematous base, dysuria (likely due to irritation of the ulcers), tender bilateral lymphadenopathy (common with primary genital HSV infection), and systemic symptoms (eg, fever, headache) in the setting of a new sexual partner.
The appearance of genital HSV lesions can vary and mimic other disease processes as the lesions change from vesicles to ulcers. Therefore, a suspected clinical diagnosis of genital HSV requires laboratory confirmation via PCR, viral culture (low sensitivity, particularly as lesions heal), direct fluorescence antibody testing, or Tzanck smear (showing multinucleated giant cells).
Question

B. Fluticasone
This patient’s autopsy findings of lung hyperinflation and bronchial inflammation are suggestive of uncontrolled asthma, a disease characterized by chronic airway inflammation, airway hyperresponsiveness, and intermittent bronchoconstriction. Chronic inflammation, composed mainly of eosinophils, helper T cells, and mast cells, causes airway remodeling (ie, bronchial wall thickening, increased smooth muscle), which further worsens airway obstruction and asthma symptoms.
Corticosteroids inhibit the production of inflammatory mediators (eg, cytokines, prostaglandins, leukotrienes), reduce leukocyte extravasation into the respiratory epithelium, and induce apoptosis of inflammatory cells. In addition, corticosteroids decrease smooth muscle proliferation and mucus production by goblet cells.
Systemic corticosteroids (eg, oral prednisone) are used in short courses to treat acute asthma exacerbations, whereas inhaled corticosteroids (eg, fluticasone) reduce the frequency and severity of exacerbations and are used for long-term asthma control in patients with persistent symptoms. Suppression of airway inflammation is evident within hours of administration but reaches maximal effect after several months of inhaled therapy. Nonadherence to long-term therapy can increase the risk of life-threatening asthma exacerbation.
Question

A. Decreased Cholecystokinin release due to lack of enteral stimulation

Cholesterol is secreted in bile, where it is solubilized by bile salts and phosphatidylcholine. If there is more cholesterol than can be dissolved by the bile salts, the cholesterol will precipitate into insoluble crystals, leading to the formation of gallstones. Risk factors for gallstone formation include obesity or rapid weight loss, female sex, glucose intolerance, and hypomotility of the gallbladder (eg, pregnancy, prolonged fasting).
A prolonged course of total parenteral nutrition (TPN) is often complicated by gallstones. In normal individuals, enteral passage of fat and amino acids into the duodenum triggers release of cholecystokinin (CCK), leading to contraction of the gallbladder. The absence of normal enteral stimulation in patients receiving TPN leads to decreased CCK release and subsequent biliary stasis. In addition, patients with extensive resection of the ileum can have disruption to the normal enterohepatic circulation of bile acids, leading to inadequate solubilization of biliary cholesterol and formation of cholesterol crystals.
A 62-year-old man comes to the office due to an intensely pruritic facial rash for the past 3 days. The patient uses no facial cosmetic products but has frequently dyed his hair during the past year; he last dyed his hair 5 days ago and also recalls developing a similar rash the previous time he used hair dye. The patient has a history of asthma, hypertension, and diabetes mellitus. He does not use tobacco, alcohol, or illicit drugs. Vital signs are within normal limits. Physical examination findings are shown in the exhibit. Which of the following are primarily involved in the pathogenesis of this patient’s rash?

CD8+ T cells and INF gamma

This patient developed erythema and pruritus on the scalp, face, and neck 2 days after reexposure to hair dye. Hair products, including dye, frequently contain allergenic molecules (eg, p-phenylenediamine) that can cause allergic contact dermatitis (ACD). ACD is a type IV hypersensitivity (delayed-type) reaction that occurs in 2 phases:
Sensitization: Cutaneous Langerhans cells take up haptens (small molecules that bind to proteins and alter their immune appearance) and present hapten-peptide complexes to naive CD4+ and CD8+ T cells in regional lymph nodes, resulting in clonal expansion of hapten-sensitive T cells. This phase takes 10-14 days and does not result in cutaneous lesions.
Elicitation: On reexposure to the hapten, sensitized T cells are recruited to skin for activation by hapten-protein conjugates displayed on cutaneous antigen-presenting cells. Activated CD8+ T cells, the main effector cells in ACD, release cytotoxins (eg, perforin, granzymes) and express Fas ligand to induce keratinocyte apoptosis. They also amplify the inflammatory response by releasing cytokines (eg, interferon gamma) and recruiting additional inflammatory cells (eg, macrophages). This phase occurs 2-3 days following reexposure to the hapten and results in erythema, pruritus, and vesicles.
Question

C. IL-4
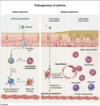
There are 2 classes of CD4+ T-helper cells, Th1 cells and Th2 cells. Th1 cells contribute to cell-mediated adaptive immunity (targeting intracellular pathogens) and type IV (delayed-type) hypersensitivity reactions. On the other hand, Th2 cells play a prominent role in allergic response and type I hypersensitivity reactions.
One hypothesis for the pathogenesis of asthma is an excess of Th2 cell activity relative to Th1 cell activity, resulting in excessive IgE production, an abnormal propensity for type I hypersensitivity reactions, and associated chronic eosinophilic bronchitis. In the asthma sensitization phase, inhaled antigens stimulate Th2 cells to secrete IL-4 and other lymphokines to stimulate B-cell antibody production as part of humoral adaptive immunity. Th2 cells also release IL-13, which, together with IL-4, promotes B-cell immunoglobulin class switching to IgE and leads to mast cell priming.
Repeat exposure to inhaled antigens leads to mast cell degranulation of inflammatory substances (eg, histamine, leukotrienes) and further activation of eosinophils with release of tissue-damaging substances (eg, major basic protein).
(Choice D) IL-5 is secreted by activated Th2 cells and stimulates the growth and differentiation of eosinophils. However, IL-5 promotes the class switching of B-cell immunoglobulin synthesis to IgA rather than to IgE.
Question

A. Acid alpha glucosidase
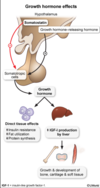
This patient most likely has glycogen storage disease type II (Pompe disease). This condition is caused by deficiency of acid alpha-glucosidase (alpha-1,4 glucosidase or acid maltase), an enzyme responsible for breaking down glycogen within the acidic environment of lysosomes. Although most glycogen is degraded in the cytoplasm, a small percentage is inadvertently engulfed by lysosomes, especially in cells containing high amounts of glycogen such as hepatocytes and myocytes. Deficiency of acid maltase results in pathologic accumulation of glycogen within liver and muscle lysosomes. Cardiac and skeletal muscle are particularly susceptible because the ballooning lysosomes interfere with contractile function.
The classic form of the disease presents in early infancy with marked cardiomegaly, severe generalized hypotonia, macroglossia, and hepatomegaly. Blood glucose levels are normal, unlike with glycogen storage diseases that primarily affect the liver (eg, von Gierke disease). A key distinguishing feature is that muscle biopsy will show accumulation of glycogen in lysosomes.
Type II Pompe Disease pathophyisiology
This condition is caused by deficiency of acid alpha-glucosidase (alpha-1,4 glucosidase or acid maltase), an enzyme responsible for breaking down glycogen within the acidic environment of lysosomes. Although most glycogen is degraded in the cytoplasm, a small percentage is inadvertently engulfed by lysosomes, especially in cells containing high amounts of glycogen such as hepatocytes and myocytes. Deficiency of acid maltase results in pathologic accumulation of glycogen within liver and muscle lysosomes. Cardiac and skeletal muscle are particularly susceptible because the ballooning lysosomes interfere with contractile function.
The classic form of the disease presents in early infancy with marked cardiomegaly, severe generalized hypotonia, macroglossia, and hepatomegaly. Blood glucose levels are normal, unlike with glycogen storage diseases that primarily affect the liver (eg, von Gierke disease). A key distinguishing feature is that muscle biopsy will show accumulation of glycogen in lysosomes.
Acid alpha-glucosidase deficiency
Type 2 Pompe Disease
Key features of Pompe Disease
Severe Cardiomegaly
glycogen accumulation in lysosomes
Normal Glucose Levels
Glucose-6-phosphatase deficiency
Type 1 Von Gierke Disease
Key features Type 1 Von Gierke Disease
Hepatomegaly
Steatosis
Fasting hypoglycemia
Lactic acidosis
Hyperuricemia
hyperlipidemia
Type 3 Cori Disease pathophysiology
Deficiency of Debranching Enzyme (alpha-1, 6-glucosidase)
or
Debranching Enzyme (alpha-1, 4-transferase)
Debranching enzyme (alpha-1, 6-glucosidase) deficiency
Type 3 Cori Disease
Key features of Type 3 Cori Disease
Hepatomegaly
Ketotic hypoglycemia
Hypotonia & weakness
abnormal glycogen with very short outer chains
Debranching Enzyme (alpha-1, 4-transferase) Deficiency
Type 3 Cori Disease
Glycogen Phosphorylase deficiency
Type 5 McArdle Disease
Type 5 McArdle Disease pathophysiology
Glycogen phosphorylase Deficiency
Key Features Type 5 McArdle Disease
Muscle Phosphorylase deficiency
Weakness & fatigue with exercise
No rise in blood lactate levels after exercise
Galactokinase Deficiency
Galactokinase catalyzes the phosphorylation of galactose to galactose-1-phosphate in the first committed step of galactose catabolism. Galactokinase deficiency causes neonatal cataract formation due to accumulation of galactitol in the lens
Question

F. Sphingomyelin
Niemann-Pick disease is an autosomal recessive disorder common among Ashkenazi Jews and is characterized by sphingomyelinase deficiency. Sphingomyelinase is responsible for the breakdown of sphingomyelin, a lipid constituent of cell membranes. In Niemann-Pick disease, sphingomyelin accumulation within lysosomes results in cells that appear enlarged, foamy, and vacuolated on electron microscopy. These lipid-laden foam cells accumulate in the liver and spleen and cause hepatosplenomegaly. Progressive neuronal accumulation is responsible for hypotonia and neurologic degeneration (eg, failure to progress developmentally, loss of milestones). Retinal accumulation leads to a cherry-red macular spot.
This patient is presenting with the classic infantile type A variant that results in hepatosplenomegaly, progressive neurologic deterioration, and death by age 3 years.
(Choice E) Patients with Tay-Sachs disease are deficient in β-hexosaminidase A, which leads to GM2 accumulation in neurons. Neurologic regression and cherry-red macular spots are classic symptoms (similar to Niemann-Pick disease), but hepatosplenomegaly is absent.
Niemann Pick Disease Pathophysiology
Niemann-Pick disease is an autosomal recessive disorder common among Ashkenazi Jews and is characterized by sphingomyelinase deficiency.
Sphingomyelinase is responsible for the breakdown of sphingomyelin, a lipid constituent of cell membranes. In Niemann-Pick disease, sphingomyelin accumulation within lysosomes results in cells that appear enlarged, foamy, and vacuolated on electron microscopy. These lipid-laden foam cells accumulate in the liver and spleen and cause hepatosplenomegaly. Progressive neuronal accumulation is responsible for hypotonia and neurologic degeneration (eg, failure to progress developmentally, loss of milestones). Retinal accumulation leads to a cherry-red macular spot.

Niemann Pick Histology
sphingomyelin accumulation within lysosomes results in cells that appear enlarged, foamy, and vacuolated on electron microscopy

Identify


Question

C. Diffuse Atelectasis

This preterm infant with increased work of breathing and hypoxia has diffuse ground-glass opacities and air bronchograms on imaging. These findings are consistent with neonatal respiratory distress syndrome (RDS). RDS is caused by immaturity of type 2 pneumocytes, which normally produce alveolar surfactant. Lack of surfactant causes decreased compliance and increased surface tension of alveoli, leading to alveolar collapse at the end of expiration. This diffuse atelectasis results in the characteristic reticular or ground-glass opacities on chest x-ray. Unlike alveoli, larger airways remain patent and filled with air due to their cartilaginous walls, making them visible (air bronchograms) against the reticular background.
Management of RDS is respiratory support (to maintain alveolar pressure and prevent collapse) and surfactant (to reduce surface tension). During the first week of life, type 2 pneumocytes begin to release endogenous surfactant, and respiratory distress typically begins to improve.