Unit 1-Acid/Base Equilibria Flashcards
(30 cards)
a) In water and aqueous solutions, there is an equilibrium between which substances?
b) What is the dissociation equation for the above equilibrium?
c) What is the equation for calculating the dissociation constant for the above equilibrium?
a) There is an equilibrium between the water molecules and hydronium (hydrogen) and hydroxide ions.
b) 2H2O ⇌ H3O+ + OH-
H2O ⇌ H+ + OH-
c) Kw = [H+][OH<span>-</span>]
In data booklet
The data booklet states the value of the equilibrium constant/ionic product of water at 24 ºC is 10−14
What must the concentrations of hydrogen and hydroxide be at equilibrium?
Kw = [H+][OH-]
10-14 = [H+][OH-]
Both must have a concentration of 10-7 moll-1
Which equation shows the relationship between pH and the hydrogen ion concentration?
pH = -log10[H+]
[H+] = 10-pH
What are the Brønsted-Lowry definitions of acids and bases?
An acid is a proton donor
A base is a proton acceptor
What is formed when an acid loses a proton?
What is formed when a base gains a proton?
Conjugate base
Conjugate acid
What is the difference between strong and weak acids/bases?
Strong acids/bases are completely dissociated into ions in aqueous solutions
Weak acids/bases are partially dissociated into ions in aqueous solutions
Give an example of a
(a) strong acid
(b) weak acid
(c) strong base
(d) weak base
(a) Hydrochloric acid, sulfuric acid or nitric acid
(b) Ethanoic acid, carbonic acid and sulfurous acid
(c) Solutions of metal hydroxides
(d) Ammonia and amines
Compare equimolar weak and strong acids in terms of:
- pH values
- Conductivity
- Reaction rates
- Stoichiometry
- Strong acids have lower pH (more hydrogen ions in solution)
- Strong acids have higher conductivity (more ions in solution)
- Strong acids react faster (more hydrogen ions in solution)
- Both will react with the same number of molecules of base
Explain why the volume of alkali required to neutralise a strong and a weak acid of the same concentration is the same.
Use ethanoic acid as the weak acid in this example.
CH3COOH ⇌ H+ + CH3COO-
H+ + OH- → H2O
The hydroxide ions in the alkali react with all of the available hydrogen ions in solution. However, in a weak acid, this removes hydrogen ions from the equilibrium and causes the acid molecules to release more hydrogen ions. This continues until all the acid molecules have dissociated, i.e. until the acid is neutralised.
The acid dissociation constant is represented by
Ka =?
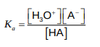
How can you convert Ka to pKa and vice versa?
pKa = -log10Ka
Ka = 10-pKa
How do you calculate the approximate pH of a weak acid?
pH = 1/2pKa - 1/2log10c
A soluble salt of a strong acid and a strong base dissolves in water to produce what type of solution?
Neutral
A soluble salt of a weak acid and a strong base dissolves in water to produce what type of solution?
Alkaline solution
A soluble salt of a strong acid and a weak base dissolves in water to produce what type of solution?
Acidic solution
Explain why a solution of ammonium chloride is acidic.
The solution will contain NH4+ and Cl- from the salt and H<span>+ </span>and OH<span>-</span> ions from the water.
Some of the NH4+ and OH<span>- </span>will react to form an equilibrium mixture of ammonium hydroxide.
This will leave an excess of H<span>+</span> ions and therefore the solution will be acidic.
Explain why a solution of sodium carbonate is basic.
The solution will contain Na+ and CO32- from the salt and H<span>+ </span>and OH<span>-</span> ions from the water.
Some of the H+ and CO32-<span> </span>will react to form an equilibrium mixture of carbonic acid.
This will leave an excess of OH- ions and therefore the solution will be basic.
What does a buffer solution do?
The pH of a buffer solution remains approximately constant when small amounts of acid, base or water are added.
How is an acid buffer made?
A solution of a weak acid and one of its salts made from a strong base.
How does an acid buffer work?
The weak acid provides the hydrogen ions when these are removed by the addition of a small amount of base.
If an acid (H+) is added the conjugate base will form the weak acid.
How is a basic buffer made?
Solution of a weak base and one of its salts.
How does a basic buffer work?
The weak base removes excess hydrogen ions,
The salt of this base provides the conjugate acid which combines with the hydroxide ions added.
Which equation can be used to calculate the approximate pH of an acid buffer solution?

What are indicators?
Weak acids which in aqueous solutions the colour of an acid indicator is distinctly different from that of its conjugate base.


