Test 2- Circulatory Diseases Flashcards

Pulmonary congestion: Usually the result of heart failure and associated with edema.
can see due to bright red color

Diffuse brownish discoloration of the lungs of a dog with chronic pulmonary edema and congestion secondary to left-sided CHF, Noah‟s Arkive

Pulmonary hemosiderosis
presence of “heart failure cells).
liquid in the cytoplasm

SUBACUTE TO CHRONIC HEPATIC CONGESTION IS USUALLY THE RESULT OF RIGHT-SIDED CHF
Livers are enlarged and exhibit rounded edges

Chronic hepatic congestion: “Nutmeg liver”

Subacute hepatic
Congestion – “Nutmeg Liver
Chronically there is low-grade Hypoxia & ↑ pressure of centrolobular hepatocytes leading to atrophy and necrosis.

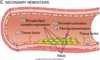
Vascular Endothelium
Role in hemostasis
Anti-thrombotic & pro-fibrinolytic in the normal state
Pro-thrombotic and anti-fibrinolytic during injury
Modulates perfusion:
NO relaxes and causes vasodilation
Endothelin causes vasoconstriction
Role in inflammation:
Regulates the traffic of inflammatory cells
Produces pro-inflammatory cytokines
Control angiogenesis and tissue repair
Fluid distribution
Total BodyWater:
65% of total body weight Plasma (5%)
Interstitial Fluid (15%)
Intracellular Fluid (40%)
Transcellular Fluid (5%)
YOUNGER INDIVIDAULS HAVE A LARGER WATER CONTENT
Homeostasis:
“A tendency to stability in the normal body states”
Interstitium
Space between tissue compartments (microcirculation and the cells).
Is the medium through which all metabolic products must pass between the microcirculation and the cells.
Composed of the Extracellular Matrix (ECM) and supporting cells
Extracellular Matrix
Composed of structural molecules (collagen, reticulin, elastic fibers) and ground substance (glycoproteins like fibronectin & laminin, plus glycosaminoglycans, proteoglycans etc..)
Water distribution between plasma & the interstitium is primarily determined by
Water distribution between plasma & the interstitium is primarily determined by the hydrostatic & osmotic pressures differences between the 2 compartments
Starling Forces:
In simple terms, the hydrostatic pressure moves fluid out of the vasculature; the osmotic pressure of plasma proteins (oncotic pressure) moves fluid into the vasculature.
however, if the capacity for lymphatic drainage is exceeded
however, if the capacity for lymphatic drainage is exceeded, tissue edema results
Edema
–Abnormal accumulation of excess extracellular water in
interstitial spaces or in body cavities
– Fluid is outside both the vascular fluid compartment and cellular fluid compartment (i.e.: within the interstitium).
Pathomechanisms of Edema
1. Increased blood hydrostatic pressure (Generalized: e.g.right-sided congestive heart failure (CHF); Localized: e.g.: tightly bandaged limb resulting in venous occlusion.
- *2. Decreased plasma colloidal osmotic (a.k.a. oncotic) pressure**
- –Proteins not absorbed from diet (e.g.: starvation, GI malabsorption).
- –Proteins not produced (e.g.: liver disease)
- –Protein loss (e.g. glomerular disease, Intestinal mucosal damage)
3. Lymphatic obstruction. Damage/ obstruction of lymphatics (e.g.: surgery, neoplasms, inflammation)
4. Increased vascular permeability (Inflammation)
Edema can also be classified as
Edema can also be classified as “inflammatory” or “non-inflammatory” edema.
Inflammatory Edema
Inflammatory: Increased vascular permeability – refers as an “exudate”
Edema fluid in these cases is “protein rich” an exudate
(high protein content (>30g/L), specific gravity (>1.025), total nucleated cells (<7x109L)less than 7,000 cells per μl.
Non-inflammatory Edema
Non-inflammatory (e.g.: edema of CHF; edema of liver failure) – refers to as a “transudate”
Edema fluid in these cases is “protein poor” low protein content (<30g/L), low specific gravity (<1.025), low cellularity (<1.5x109L) less than 1,500 cells per μl.

Gross Appearance of Edema:
Wet
Gelatinous and heavy
Swollen organs
Fluid weeps from cut surfaces
May be yellow

Histological appearance of edema
Clear or pale eosinophilic staining depending on whether is non-inflammatory or inflammatory edema.( inflammatory is pink because it has a high protein content)
Spacesaredistended
Blood vessels may be filled with
red blood cells
Lymphaticsaredilated
Collagenbundlesareseparated


Pitting edema
When pressure is applied to an area of edema a depression or dent results as excessive interstitial fluid is forced to adjacent areas
- takes a while for the tissue to go back to normal after you press in

Hydrothorax: fluid in the thoracic cavity
Heifer, Hydrothorax (idiopathic pulmonary hypertension)

Pericardial effusion – “mulberry heart disease”- (inflammatory edema). Note fibrin strands and cloudy appearance of the pericardial fluid.
ass with Vit E/selinum deficiency

Ascites or hydroperitoneum: fluid (transudate) within the peritoneal cavity. Dog with CHF. From McGavin, 2007.