Pharmacology L3: Adverse Drug Reactions Flashcards
What is the goal of drug therapy?
The goal of drug therapy is to select a dose in the therapeutic range and to avoid doses that produce toxicity.
This is not an easy process, as many factors affect how much drug reaches the site of action, such as route of _____, _____ and drug ______.
administration; absorption; metabolism
Genetics, age, gender…etc
What is the Drug Pipeline: Research and Development through to use by patients?

Even after this pipeline has been completed and the drug is being used by patients, the numbers of ADRs which occur after commercial release may be very small for an individual doctor, but when captured across a country or globally, trends may appear

What are Adverse Drug Reactions (ADR)?
“any response to a drug which is noxious (Unpleasant), unintended, and which occurs at doses normally used for therapy of disease”
Serious ADRs should be reported to a _____ to build up evidence across all users over time. (Safety concerns –> observe the effects) Drugs are withdrawn from the market each year as a result of such data. (Can change drugs) This definition does not cover drug overdose, drug abuse, or error in administration of drug.
national database
How does drug safety done?
Federal policies govern the approval and use of all drugs
All drugs must satisfy two major requirements before it can be approved in humans. What are they?
- Efficacy (effectiveness) in the disease state for which it is approved
- Safety, determined by extensive animal testing and controlled human testing.
What are the 6 various types of ADRs?
The most frequently used scheme for classifying types of ADRs is simply: ABCDEF
- Type A: Acute, predictable, related to MOA or ADME
- Type B: “Bizarre”, unpredictable, not necessarily related to MOA, but may involve patient qualities
- Type C: Chronic (continuous) effects
- Type D: Delayed effects
- Type E: End of treatment effect
- Type F: ???
What is type A (acute) for ADRs?
Dose-related toxicity

What is type A (acute) for ADRs related to?
- Related to the main pharmacological action of the drug, and often dealt with by reducing the dose
- Should be evident from clinical trials, reproducible in animals;

Type A (acute) Dose-related toxicity is generally ______ (predictable/unpredictable) and is ______ -dependent.
predicable; concentration

What are specific groups of patients that many be at risk for type A (acute) dose-related toxocity?
e.g. elderly, patients with kidney/liver damage, young children

What is phenytoin, an anticonvulsant used for epilepsy?
- drug given at different doses per individual (x-axis) blood concentrations measured
- <10 μg/ml is lower than the therapeutic range (reduced or no effectiveness)
- >15 μg/ml begin to show toxic (adverse) effects Note the large amount of individual variation shown by patients!
- A narrow therapeutic window requires careful patient monitoring as concentrations are a key determining factor. Thus, patients would have to submit to blood testing to check the concentration present.

What is the blood concentrations that is lower than the therapeutic range (reduced or no effectiveness) for phenytoin, an anticonvulsant used for epilepsy?
<10 μg/ml

What is the blood concentrations begin to show toxic (adverse) effects for phenytoin, an anticonvulsant used for epilepsy?
>15 μg/ml

What is the Therapeutic window?
range of concentrations where therapeutic effect is obtained without toxicity.

What is the Therapeutic Index?
ratio of desired vs adverse effect; the larger the better.

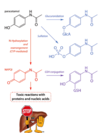
How is drug metabolism also a significant contributor to Type A ADRs?
- Example: An antihistamine, SeldaneR (terfenadine) was being used in the USA by prescription. It had an advantage over other antihistamines in that it did not cause drowsiness as it did not cross the blood-brain barrier. It was a prodrug, with the enzymes converting it to fexofenidine.
- Initial testing in the lab and in patients (pipeline) had no ADRs. There were no significant safety issues associated with terfenadine.
- However, it was used by 24 million patients for more than a decade – and one ADR that was detected in a few patients without any cardiovascular problems were going to hospitals with potentially fatal arrhythmias.
What is the Terfenadine’s novel MOA?
Research as to terfenadine’s effects on the heart showed that it had the ability to block specific K+ ion channels of the heart (HERG), but at concentrations higher than expected if the patient was using the antihistamine at the recommended dose.

Research as to terfenadine’s effects on the heart showed that it had the ability to block specific K+ ion channels of the heart (HERG), but at concentrations _____(higher/lower) than expected if the patient was using the antihistamine at the recommended dose.
higher

How were higher concentrations possible in Terfenadine’s novel MOA?
- Attention turned to the metabolism of terfenadine. We knew that it was metabolised by CYP3A4 in the liver.
- It was found that patients who were taking other medications at the same time as terfenadine were most likely to have issues….particularly those which also used CYP3A4! Free to travel in blood stream at higher conc. = adverse effects of K+ ion channels of heart = heart arrhythmia

What is the problem with Terfenadine?
- This discovery was not obvious during clinical trials – it was only really determined that there was an effect on the heart that was dangerous once terfenadine was used in the marketplace with millions of people, combining with other drugs.
- Terfenadine is NOT on the market any longer – instead, its metabolite (fexofenadine)and other similar drugs are – after being screened for HERG effects.

What are 2 good examples of how the actions of one drug can affect the metabolism of another?
- Competitive inhibition of P450s
- Potent inhibition of a P450 by a single drug