Nichols Miscellany Flashcards
(63 cards)
How would you characterize classic cellular heart transplant rejection? What is another mechanism of injury?
-
Interstitial lymphocytic inflammation with associated myocyte damage; histology resembles myocarditis
1. May also be interstitial edema due to vascular injury; local cytokines can impact myocardial contractility w/o eliciting myocyte damage - Increasingly, Ab-mediated rejection also recognized as patho mechanism of injury; donor-specific Abs against MHC proteins lead to complement activation and recruitment of Fc-receptor-bearing cells
1. Mild rejection may resolve spontaneously, while prompt recognition of more severe cases allows successful treatment by INC baseline levels of immunosuppression; aggressive anti-T cell or anti-B cell immunotherapy, w/or w/o plasmapheresis may be necessary
What is coronary artery hyper-reactivity?
- Aka, cardiac Raynaud: coronary artery vasospasm due to blood vessel hyper-reactivity -> can also cause myocardial contraction band necrosis
- Can cause a syndrome of chest pain identical in quality to the chest pain from atherosclerotic coronary artery disease (angina pectoris)
What is Trousseau syndrome?
- Hypercoagulable state resulting from venous thrombi generated via elaboration of procoagulant factors from malignant tumors
- Can manifest as evanescent thromboses in different vascular beds at different times
- Aka, migratory thrombophlebitis
What are the symptoms and signs of cardiac myxoma?

- Symptoms: dyspnea +/- orthopnea, cough +/- hemoptysis, fatigue, fever, transient neuro symptoms +/- syncope (fainting)
- Signs: loud first heart sound, diastolic rumble, diastolic tumor plop, holosystolic murmur (begins at first heart sound and continues to second)
- NOTE: other things that might cause a holosystolic murmur include ventricular septal defect or mitral regurgitation
What is Sturge-Weber syndrome?

- Aka, encephalotrigeminal angiomatosis
- Uncommon congenital disorder associated with:
1. Facial port wine nevi
2. Ipsilateral venous angiomas in the cortical leptomeninges
3. Mental retardation, seizures
4. Hemiplegia (partial paralysis of one side of the body), and radiopacities of the skull
What is a cardiac myxoma?
- Benign gelatinous mesenchymal neoplasm of the endocardium
- Rare
- More common in females
- Most in left atrium
What do you see?

- Normal myocardium
- Central nuclei
- Lipofuscin wear and tear pigment around the nuclei in older adults
- Cytoplasmic cross-striations
What do you see?

- Kaposi sarcoma
- Dilated, irregular endothelial cell-lined vascular spaces
What is the epidemiology of DVT?
- Rates higher in men than women, and increase with age
- Most cases of secondary VTE associated with more than one underlying condition, incl: cancer, major trauma, hospitalization, and surgery
- No antecedent trauma, surgery, immobilization, or diagnosis of cancer in 48% (in the study referenced for these numbers)
Describe cardiac muscle structure.

- Intercalated discs are anchoring structures containing gap junctions
- Cardiac muscle cells: faintly striated, branching, mononucleated cells that connect via intercalated discs to form a functional network
- Action potential travels through all cells connected together, forming a functional synctium in which cells function as a unit
- NOTE: they all need to pull together at exactly the same time to be effective
How might one diagnose DVT?
- Erythema, superficial venous dilation not helpful
- Calf/ankle swelling more sensitive, but not very specific; swelling of entired leg more specific, but not so sensitive
- Measure calf differences: asymmetry over 2cm = positive screening for DVT
- Homan’s sign: forcefully dorsiflex foot; calf pain = + screening test for DVT (WARNING: manipulating leg can dislodge thrombus, embolizing it to lung)
- D-dimer: in pts w/a low clinical risk of DVT, negative D-dimer has negative predictive value of 99%
What do you see here?

-
Rhabdomyoma: gray-white myocardial masses that can be small or up to several cm in diameter
1. Usually multiple; preferentially involve ventricles, protruding into lumen - Microscopically: composed of bizarre, markedly enlarged myocytes; routine histologic processing often artifactually reduces abundant cytoplasm to thin strands that stretch from nucleus to surface membrane, an appearance called SPIDER CELLS
What is the pathogenesis of Kaposi sarcoma?
- HHV8, aka Kaposi sarcoma herpesvirus (KSHV)
1. HHV8 is transmitted sexually, & via other poorly understood nonsexual routes potentially incl. oral secretions and cutaneous exposures - HHV8, altered T-cell immunity likely required for KS
- Devo and progression tightly linked to immune func
- Virally encoded G protein induces VEGF production, viral homologue of cyclin D drives proliferation, and multiple KSHV-encoded proteins inhibit p53
What is SVC syndrome?

- Usually caused by neoplasms that compress or invade the superior vena cava, such as bronchogenic carcinoma or mediastinal lymphoma
- Resulting obstruction produces a characteristic clinical complex consisting of marked dilation of the veins of the head, neck, and arms associated with cyanosis
What do you see here?

- EARLY heart transplant allograft arteriopathy
- Infiltration of coronary artery tunica intima by T-lymphos that bind endo cells, recognize them as foreing attack and elicit inflammation
- Thickens the intima, mostly sparing the media and adventitia -> contrast to autoimmune vasculitis, which is usually transmural
What do you see?

- Kaposi sarcoma
- Sheets of plump, proliferating spindle cells with marked hemosiderin deposition
What are the most frequent metastatic tumors involving the heart? How do they get there?
- > Carcinomas of the lung and breast
- > Melanomas
- > Leukemias and lymphomas
- Metastases can reach the heart and pericardium by retrograde lymphatic extension (most carcinomas), by hematogenous seeding (many tumors), by direct contiguous extension (primary carcinoma of the lung, breast, or esophagus), or by venous extension (tumors of the kidney or liver)
What is the epi and pathology of rhabdomyomas?
- Most frequent primary tumor of pediatric heart, and commonly discovered in first years of life because of obstruction of a valvular orifice or cardiac chamber
- About 50% due to sporadic mutations; other 50% associated with tuberous sclerosis, with mutations in the TSC1 or TSC2 tumor suppressor gene.
1. TSC1 and TSC2 proteins (hamartin & tuberin) function in a complex that inhibits activity of the mammalian target of rapamycin (mTOR), a kinase that stimulates cell growth and regulates cell size -> expression often absent in tuberous sclerosis-associated rhabdomyomas, providing a mechanism for myocyte overgrowth - Occur spontaneously, so may be considered hamartomas rather than true neoplasms
What is hereditary hemorrhagic telangiectasia?
- Aka, Osler-Weber-Rendu disease: auto dom; caused by muts in genes that encode components of TGF-β signaling pathway in endo cells
- Telangiectasias present at birth, and widely distributed
- Lesions can spontaneously rupture, causing serious epistaxis (nosebleed), gastrointestinal bleeding, or hematuria
How is KS treated?
- Course of KS varies widely, and is significantly influenced by the clinical setting
- Most primary KSHV infections are asymptomatic
- Classic KS: initially restricted to body surface, and sx resection usually adequate for excellent prognosis; radiation can be used for multiple lesions in restricted area, and chemo yields satisfactory results for more disseminated disease, incl. nodal involvement
- Immunosupp KS: withdrawal of immunosuppression (with adjunct chemo or radiotherapy) often effective
- HIV KS: antiretroviral therapy has greatly DEC freq of this type of KS, emphasizing central role that T cell immunodeficiency has in the disease
- Interferon-alpha and angiogenesis inhibitors are variably effective; newer strategies aimed at specific kinases downtream of VEGF receptors show promise
What is DVT?
- Deep vein thrombosis
- Common: est. 400,000 per year in the US
- Serious: can be fatal when embolizes (6% of pts who present with DVT die of embolism w/in 1 month of presenting)
- Pts who present w/DVT + those who present w/PE = venous thromboembolism (VTE)
1. Deaths due to VTE est. at 100,000/yr in US
What are venous stasis ulcers? How are arterial ulcers different?

- Chronic venous disease is the most common cause of lower extremity ulcers
- Usually low on medial ankle over perforating vein, or along course of great or small saphenous veins; never in the forefoot or above the level of the knee
- May be multiple or single, and tender, shallow, exudative with granulation base -> borders usually irregular, but not undermined
- Arterial ulcers typically painful, and punched out or stellate in appearance
- Surrounding skin red, taut; some arterial ulcers pale, but others may have black, yellow eschar
What is portal vein HTN?
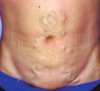
- Caused by liver cirrhosis (and less frequently, portal vein obstruction or hepatic vein thrombosis)
- Leads to opening of porto-systemic shunts and increased blood flow into veins at:
1. Gastro-esophageal junction (esophageal varices): most important bc prone to ruptures that can lead to massive (even fatal) upper GI hemorrhage
2. Rectum, forming hemorrhoids
3. Periumbilical veins of abdominal wall: caput medusae
What is going on here?

- LATE heart transplant allograft arteriopathy
- Severe intimal thickening by mostly fibrous tissue
- Notice (1) how concentric it is and (2) how little atheroma there is -> contrast to typical eccentric atherosclerotic plaque





















