04.06 and 04.07 - Atherosclerosis Flashcards
(50 cards)
What is atherosclerosis, and its epi?
- Disease of arterial intimal thickening due to lipid accumulation, chronic inflammation, repair response
- Involves medium (muscular) and large (elastic) aa
- Coronary atherosclerosis -> MI, cerebral -> cerebral infarction, and leg involvement gangrene
- Universal prevalence in adults; serious (causes half of deaths; MI ¼ of deaths)
- Typically middle age (incidence of MI INC 5-fold b/t age 40 and age 60, 10 years earlier in men)
How do risk factors affect atherosclerotic CV events?
- 80% of atherosclerotic CV events, like infarctions, occur in ppl with risk factors
- Major risk factors multiplicative rather than additive
- Subdivide into “constitutional,” like age and sex, and modifiable, like high chol, HTN, smoking, diabetes, inflammation, high BP, physical inactivity, etc.
SHODDY
- Smoking
- HTN
- Obesity: 2x risk of MI, 100x risk of leg gangrene
- Diabetes
- Dyslipidemia: low HDL, high LDL
- Inflammation also an independent predictor (i.e., high serum C-reactive protein)
- Physical inactivity also a predictor, but obesity is a stronger independent risk factor
What are the 3 major concepts of atherosclerosis?
- Atherosclerosis is a chronic inflammatory disease
- Obesity contributes to atherosclerosis partly by being a pro-inflammatory and pro-thrombotic state
- Atherosclerosis is a disease of response to endo cell injury
What are the 5 steps in the pathogenesis of atherosclerosis?
- Endo injury and dysfunction -> increased vascular permeability and leukocyte, platelet adhesion to endo
- Accumulation of lipoproteins (mainly LDL and its oxidized forms) in the intima
- Monocyte migration into intima and transformation into macrophages and foam cells
- Factor release from activated platelets, macros, and vascular wall cells -> smooth muscle cell recruitment, from the media, and recruitment of T cells*
- Smooth muscle cell proliferation, ECM production
*NOTE: T cells important in later complicated athero
What are the dominant lipids in atheromatous plaques?
Cholesterol and cholesterol esters
How are plasma cholesterol and LDL implicated?
- Genetic defects in lipoprotein uptake & metabolism that cause hyperlipoproteinemia are associated with accelerated atherosclerosis
1. Ex: familial hypercholesterolemia - defective LDL receptors and inadequate hepatic LDL uptake can precipitate MI before age 20 - Other genetic/acquired diseases (e.g., diabetes, hypothyroidism) that cause hyper-cholesterolemia lead to premature atherosclerosis
- Epi shows significant correlation b/t severity of atherosclerosis and levels of total plasma cholesterol or LDL
- Lowering serum chol by diet/drugs slows rate of progression of atherosclerosis, causes regression of some plaques, and reduces risk of CV events
What are the mechanisms by which hyperlipidemia contributes to atherosclerosis?
- Can directly impair endo cell function by increasing local ROS production; besides causing mem & mito damage, oxygen free radicals accelerate NO decay, dampening its vasodilator activity
- W/chronic hyperlipidemia, lipoproteins accumulate in intima, may aggregate or become oxidized by free radicals produced by inflammatory cells -> modified LDL accumulated by macros through a variety of scavenger receptors (distinct from the LDL receptor)
- Modified lipoproteins can’t be completely degraded, so chronic ingestion leads to formation of lipid-filled macros, foam cells; smooth muscle cells can transform into foam cells by ingesting modified lipids via LDL-receptor related proteins
- Modified lipoproteins toxic to endo cells, smooth muscle cells, and macros, and their binding and uptake stimulates release of growth factors, cytokines, chemokines that create a vicious cycle of monocyte recruitment and activation
How does chronic inflammation contribute to atherosclerosis?
- Triggered by accumulation of cholesterol crystals & FFA in macros & other cells -> contributes to initiation and progression of athero lesions
- Cells sense abnormal materials via cytosolic innate immune receptors (components of inflammasome); resulting inflammasome activation -> release of pro-inflam IL-1, which recruits leukocytes, incl monocytes
- T lymphos activated, but what they recognize and why substances detected as foreign invaders unkown
- Net result of macro, T cell activation local production of cyto/chemokines; recruit, activate more inflam cells
- Activated macros produce ROS that enhance LDL oxidation, and growth factors driving smooth muscle cell proliferation
- Activated T cells in the growing intimal lesions elaborate inflam cytokines, e.g., IFN-γ, which, in turn, can activate macros, endo, and smooth muscle cells; leukos and vascular wall cells release growth factors that promote smooth muscle prolif and syn of ECM proteins -> many lesions of athero attributable to the chronic inflammatory reaction in the vessel wall
How are smooth muscle cells implicated in atherosclerosis?
- Intimal smooth muscle cell prolif and ECM deposition convert fatty streak into mature atheroma, contributing to progressive growth of atherosclerotic lesions
- Intimal sm m cells have prolif, synthetic phenotype distinct from underlying medial smooth muscle cells
- Implicated growth factors in sm m prolif incl: PDGF, released by locally adherent platelets, macros, endos, and smooth muscle cells, FGF, and TGF-alpha
- These factors also stimulate smooth muscle cells to synthesize ECM (notably collagen), which stabilizes atherosclerotic plaques
- In contrast, activated inflam cells in atheromas may increase breakdown of ECM components, resulting in unstable plaques
Describe the evolution of visible wall changes in the response to injury hypothesis. NOTE: not the same as the 5 steps of pathogenesis.
- Normal
- Endo injury with monocyte and platelet adhesion
- Monocyte, smooth muscle cell migration into the intima, with macrophage activation
- Macro, smooth muscle uptake of modified lipids, with further activation and recruitment of T cells.
- Intimal smooth muscle proliferation with ECM production, forming a well-developed plaque
Why is LDL bad cholesterol?
- After accumulating in tunica intima, LDL modified by being oxidized and glycated -> subsequently trapped in tunica intima
- Modified LDL not taken up by macros via LDLr, but by a scavenger receptor -> problem is that this has no feedback inhibition and macros take up massive quantities of LDL, becoming dysfunctional foam cells, which die
What is the sequence of cellular interactions in atherosclerosis?
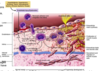
- Hyperlipidemia, hyperglycemia, HTN, other things cause endo dysfunction resulting in platelet adhesion and recruitment of circulating monocytes and T cells
- Subsequent cytokine, growth factor release (IL-1, mono chemoattractant protein-1, IFN-gamma) from inflam cells leads to smooth muscle cell migration and proliferation, & further macro activation
- Foam cells in atheromatous plaques derive from macros and smooth muscle that have accumulated modified lipids, e.g., oxidized, aggregated LDL via scavenger, LDL-receptor-related proteins
- EC lipid derived from insudation from vessel lumen, esp in presence of hypercholesterolemia, and from degenerating foam cells
- Cholesterol plaque accumulation reflects imbalance b/t influx and efflux; HDL likely helps clear cholesterol from these accumulations
- In response to elaborated cyto/chemokines, smooth muscle cells migrate to intima, proliferate, and make ECM, incl collagen and proteoglycans
What are the three principal components of atherosclerotic plaques?
- Smooth muscle cells, macros, T-cells
- ECM, incl collagen, elastic fibers, proteoglycans
- IC and EC lipid
- Note: these components present in varying proportions and configurations in different lesions
What does an atherosclerotic plaque contain?

- Typically, a superficial fibrous cap made of smooth muscle and collagen; beneath, to side of the cap (the “shoulder”) is a more cellular area w/macros, T cells, smooth muscle cells
- Deep to fibrous cap is a necrotic core, with lipid (mainy chol, chol esters), debris from dead cells, foam cells (lipid-laden macrophages, smooth muscle cells), fibrin, variably organized thrombus, and other plasma proteins; chol content frequently present as crystalline aggregates washed out in tissue processing, leaving behind only empty “clefts”
- Periphery of the lesions show neovascularization, or proliferating small blood vessels
- Most atheromas contain abundant lipid, but some plaques (“fibrous plaques”) are composed almost exclusively of smooth muscle cells and fibrous tissue
How do these images show the progression of atherosclerosis?

A. Minimal, but intima > 2 cells thick
B. Mild: some recruited monos have become foam cells, but most lipid is IC
C. Moderate: intima now much thicker than media, and pools of lipid
D. Still moderate, but cap of fibrous tissue over top of lipid (or necrotic core)
What do these stains emphasize?

- Plaques generally continue to change, progressively enlarge through cell death and degeneration, syn and degradation (remodeling) of ECM, and organization of any superimposed thrombus
- Atheromas often undergo calcification; patients with advanced coronary calcification have increased risk for coronary events
Describe the eccentricity of atherosclerosis.

- Lesions patchy, usually involve only a portion of any given arterial wall and are rarely circumferential; on cross-section, the lesions therefore appear ‘eccentric’
- Focality of atherosclerotic lesions, despite uniform exposure of vessel walls factors like cigarette smoke toxins, elevated LDL, and hyperglycemia attributable to vagaries of vascular hemodynamics
- Local flow disturbances, like turbulence at branch points, make certain portions of a vessel wall more susceptible to plaque formation
- Although focal and sparsely distributed at first, with time atherosclerotic lesions can become larger, more numerous, and more broadly distributed -> in any given vessel, lesions at various stages often coexist
How is neovascularization implicated in atherosclerosis?
- Feature of later athero
- Ingrowth of capillaries through tunica adventitia and tunica media to cells in massively thickened tunica intima, demanding vasa vasorum in an artery normally not needing them b/c oxygen, nutrients can normally get to few cells in the tunica intima by diffusion from the lumen
- These abnormal, irregular small blood vessels are prone to rupture
What does the timeline of athero devo usually look like?

-
First decade: clinically silent, and growth mainly via lipid accumulation
1. Initial lesion: histo normal, macro infiltration, isolated foam cells
2. Fatty streak: mainly IC lipid accumulation -
Third decade: clinically silent, and growth mainly via lipid accumulation
1. Intermediate lesion: IC lipid accumulation, small EC lipid pools
2. Atheroma: IC lipid buildup, core of ECM lipid -
Fourth decade: clinically silent or overt, increased sm m and collagen, thrombosis and/or hematoma
1. Fibroatheroma: single or multiple lipid cores, fibrotic/calcific layers
2. Complicated lesion: surface defect, hematoma, hemorrhage, thrombosis
Describe the role of physical disruption (i.e., rupture) of coronary arterial plaques in fatal coronary events.

- Frank rupture of fibrous cap in vulnerable plaque and superficial erosion of coronary artery may cause death
- Fibrous cap typically overlies lipid-rich center of an atheromatous plaque, standing b/t blood, with latent coag factors, and lipid core, a portion of the plaque filled with thrombogenic material
- Plaques that have ruptured and caused fatal MI often, but not always, have thin fibrous caps; ruptured plaques also tend to have large lipid cores, abundant inflam cells, and punctate or spotty calcification
- > 30% of these plaques associated with a luminal stenosis of less than 75%
How does superficial erosion of coronary atheromata cause fatal acute MI?

- Causes 20-25% of cases of fatal acute MI, more in F
- Many lack prominent inflam infiltrates and exhibit proteoglycan accumulation
- Apoptosis of endo cells -> desquamation; oxidative stress can promote endothelial apoptosis
- Hypochlorous acid (product of MPO released by activated leukocytes) can initiate endo cell apoptosis
- As these cells undergo apoptosis, they produce the procoagulant tissue factor
- Modified LDL can induce MMP-14 via endo cells, and activate MMP-2 that degrades type IV collagen
What is Lp-PLA2?
- Circulating level of this enzyme is an independent predictor of CV events
- Lipoprotein-associated phospholipase A2, produced by inflam cells, travels with circulating LDL, and hydrolyzes oxidized phospholipids in LDL
- Pro-inflammatory role mediated by products of the Lp-PLA2 reaction (lysophosphatidylcholine and oxidized nonesterified fatty acids) generated in atherosclerotic lesions -> bioactive lipids up-regulate MCP-1 (monocyte chemoattractant protein-1), ICAM-1 and VCAM-1 on endo cells, making them vasodilate less in response to NO and undergo apoptosis
- Also upregulate expression of MCP-1 on macros, making them secrete IL-1beta and undergo apoptosis
What are the critical points in the pathology of atherosclerosis?

- Vulnerable plaque has displaced luminal stenosis as critical determinant of ischemia, infarction and death (events) due to atherosclerosis (AS)
- Determinants of vulnerability are: (A) thinness of the fibrous cap, (B) size of lipid (necrotic) core, (C) amt of inflammation, and (D) amt of calcification
- Up to 75%* of AS events due to plaque rupture
- Up to 25%* of AS events are from plaque erosion
- Although atherosclerosis is a chronic inflammatory disease, events from it are most commonly due to thrombosis superimposed on it
***Don’t ignore the “up to;” there are other causes













