Ischemic Heart Disease Flashcards
What does this show?

- Gross pathology of classical MI
- Acute = light brown, tan
- Subacute = yellow
- Old = white
When is chronic rheumatic heart disease more common? About when do people typically get symptoms?
- More common with: recurrent carditis, severe carditis, and carditis at an early age
- Symptoms an average of 20 years after carditis
What are the general principles of MI pathology (i.e., gross death, infiltration, time to heal)?
- Usually takes about 12 hours for dead cardiac muscle to show macroscopic (gross) manifestations of death
- Acute inflammation (neutrophils), clean-up (macros), and repair (fibroblasts) all come in from edge ofan MI bc no blood supply within it to bring them there (that is why it infarcted)
- Bigger the infarct, the longer it takes to heal and be converted to an acellular fibrous scar -> really big one can take 3 months
What is a transmural infarction?
- Involves the full thickness of the heart wall
- 90% associated w/occlusive thrombosis super-imposed on atherosclerotic plaque with an acute change -> disruption of unstable, vulnerable plaque by rupture or erosion
What is a subendocardial infarction?
- Involving inner portion of heart wall
- More likely to be patchy, and to have episodic extension
- Becoming more common than transmural
What is acute rheumatic heart disease? What are the Jones criteria?
- Inflammation of endocardium, myocardium and epicardium (aka, pancarditis) after group A beta-hemolytic streptococcal pharyngitis
- 5 major diagnostic criteria (Jones criteria):
1. Fever
2. Polyarthritis
3. Sydenham’s chorea (rapid, uncoordinated jerking mvmts of hands and face)
4. Subcutaneous nodules
5. Erythema marginatum: pink rings on trunk and inner surfaces of limbs
What do you see here?

- Very late subacute infarct
- Almost completely converted to a scar
- Just a few lymphos left (surveying work of macros and fibros)
How do the leads help you localize an MI?
- LAD: anterior; V1-V4
- RCA: inferior; 2, 3, aVF
- LCX: lateral; 1, aVL, V5, V6
What is the mPTP? Why is it so central to mito collapse in reperfusion injury?

- Opening the mPTP undoes the mito mem potential essential for generating ATP, wrecking its ability to provide energy for the cell
- mPTP is a voltage-dependent channel regulated by Ca and oxidative stress. Three different proteins influence the function of the mPTP:
1. Voltage-dependent anion channel (VDAC): outer mem
2. Adenine nucleotide translocator (ANT): inner mem
3. Cyclophilin D (CypD): matrix side, inner mem - Together, these proteins span the 2 mito mems, providing a path from the mito matrix to cytoplasm
What do you see?

- Aschoff body
- Microscopic lesion of fibrinoid necrosis with histiocytes and Anitschkow cells (like a necrotizing granuloma, kind of)
What do you see?

- Subcutaneous nodule: one of the 5 diagnostic criteria for rheumatic heart disease (Jones criteria)
What do you see here? Why are there very few neutrophils?

- Viable myocytes with myocytolysis (aka, hibernating myocardium)
- Non-viable (dead) myocytes w/coagulation necrosis (showing loss of striations, hypereosinophilia, and loss of nuclei)
- Few polys bc they come in from the edges (where there is still blood flow; not from the subendocardial edge) -> lymphos, macros, fibroblasts that follow in the subacute healing phase also come from edges
How does reperfusion injury involve the mitochondria?
- In ischemic cardiomyocytes, lack of oxygen causes electron transport in mito to back up, priming various components of ETC to generate oxygen free radicals when oxygen returns
- With reperfusion, a form of oxidative burst provokes a massive diversion of electrons from the electron transport system to generation of oxygen radicals
- Simultaneously, a large influx of Ca occurs
- A prime target of the excess oxygen radicals and Ca is the mito permeability transition pore (mPTP), which opens mPTP, collapsing mito function -> this is a central event in ischemic reperfusion injury
What do you see?

- Hibernating myocytes: chronically ischemic myocytes that have cleared cytoplasm due to catabolism of their contractile proteins and need time to regenerate their contractile proteins before they work normally again
- Myocytolysis: light microscopic appearance of hibernating myocytes
What do you see?

- Erythema marginatum: one of the 5 diagnostic criteria for rheumatic heart disease (Jones criteria)
What is the microscopic pathology of reperfused MI?
- Subacute phase
- Days 4-10:
1. Lymphocytes (+/- eosinophils, plasma cells), then granulation tissue, collagen
2. Accelerated inflammation and repair: appears about 1 day older at 2 days, 2 at 4, and 4 at 6 - Days 11-end:
1. Healing of lg infarct can be accelerated from 12 to 7 weeks (small one done by 2 wks)
2. Patches of preserved myocardium commonly interspersed with scar -> may make re-entrant ventricular arrhythmias more common
What is the molecular basis for ischemic preconditioning?
- Begins w/activation of various G-protein coupled receptors by autocoids, incl. adenosine, bradykinin, and opioids, which are released during brief periods of ischemia and reperfusion
- Activation of these receptors initiates a complex signaling cascade, incl. multiple kinases, that leads to opening of K channels in mito mem and maintenance of mPTP and electrical potential of inner mito mem
- Preservation of mito function and ATP production is primary mech for protective effect of conditioning
What is the pathology of mitral stenosis?

- Almost all rhematic (i.e., chronic rheumatic heart disease); marked female predominance
- Slitlike fishmouth or round buttonhole stenosis with fibrous thickening and rigidity of valve
- +/- fusion of commissures
- Thickening, retraction, and fusion of chordae
What do you see?

- Acute neutrophilic response to MI
- Typically reaches max around 2 days
What do you see?

- Cardiac myocyte coagulative necrosis in narrow window after hypereosinophilia (and loss of striations, not evident here bc myocytes sectioned on end)
- Have signaled necrosis, but before neutrophils have responded to the necrosis
What is the no reflow phenomenon?
- Failure of relieving obstruction at arterial level to restore blood flow
- Attributed to microvascular obstruction or edema
How old is this infarct?
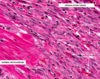
- Healing infarct (around 2-3 weeks old)
- Subacute (healing phase) MI with numerous fibroblasts and multiple new-grown blood vessels (neovascularization), which tend to come about the same time as fibroblasts, later than lymphocytes and macros
What is the reperfusion injury salvage kinase pathway?
- Protective effect of conditioning involves, among other things, activation of a reperfusion injury salvage kinase (RISK) pathway in the mitochondria
- One component of this pathway is the action of phosphatidylinositol-3 kinase (PI-3K) on Akt (protein kinase B) and mammalian target of rapamycin (mTOR)
- Other component involves mitogen-associated protein kinase (MAPK) and p42/p44 extracellular signal-related kinase (ERK)
- 2 arms of pathway converge on p70s6 kinase to activate glycogen synthase kinase beta, which acts to prevent opening of the mPTP
What are 2 reasons marantic endocarditis is a bad deal?
- Embolization from marantic endocarditis causes: strokes (cerebral infarcts w/irreperable brain losses), and infarcts of heart, kidneys, spleen, and other organs
- Worst thing: precursor for infective endocarditis











