MSK_MT1_TBL2 (Pyrimidine&Purine Metabolism) Flashcards
N10- formyltetrahydrofolate.
AA required for purine de novo synthesis
GAG
- Glycine
- Aspartate
- Glutamine
Also requires:
- Folate derivative (Vit. B9)
- CO2
Pyrminidine Synthesis requires
- glutamine
- aspartate
- tetrahydrofolate
*
Difference between Cytosine (C) & Thyamine (T)
- one methyl group

Difference between Cytosine (C) & Uracil (U)
At C-4 position, different group ( NH2 for Cystosine & C = O for Uracil)
- uracil is also only in RNA

The Pyrimidines Basic Structure

The Foundations of Pyrimidines
- Amide Donor (Glu)
- 1 C Donor (CO2 & TFH)
- 3 C & 1 N Donor (Asp)
- PRPP

Pyrimidine Synthesis: Big Picture
Goals: TO CREATE
- CMP
- UMP
- TMP
From: Ribose phosphate via HMP shunt
Key points:
- UMP synthesized first
- UMP →→→ CMP & TMP

Pyrimidine De Novo Synthesis: Step 1
Make carbamoyl phosphate via CPS-II
-
activated by PRPP and inhibited by UTP
- allosterically regulated by UTP (feedback inhibition)
- CPS-II = cytosolic
- uses Glutamine as N source
- rate-limiting step
- requires ATP

Pyrimidine De Novo Synthesis: Step 2
Make Orotic Acid
- FIRST cyclic RING of pathway produced

Pyrimidine De Novo Synthesis: Step 3
Make UMP
- Orotic Acid + PRPP = UMP
- UMP is the FIRST pyrimidine of pathway
- problems with enzyme UMP Synthase = Oroticaciduria
- due to the accumulation of Orotic Acid that cant be converted to UMP

Orotic Aciduria-UMP Synthase Deficiency
Symptoms:
- leads to orotic aciduria = ↑↑↑ orotic acid in urine
- megaloblastic anemia (large/not functional RBC)
- growth retardation
- cognitive disabilities
- renders both OPRT and OMP decarboxylase DYSFUNCT.
- no B12/Folate Response (i.e normal anemia treatment does not work… suggests UMP Synthase Deficiency)
Treatment:
- oral Uridine therapy (by pass the Synthase)
Inherited Disorders of Pyrimidine Nucleotide Metabolism
- Orotic Aciduria
-
pyrimidine 5’-nucleotidase deficiency
* ↑↑↑ blood pyrimidine ribonucleotides - OTC
Ornithine Transcarbamlyase Deficiency (OTC)
- to do with the UREA CYCLE but still makes impact
- From the UREA CYCLE, we get EXCESS of carbamoyl phosphate which gets converted into an EXCESS of Orotic acid

Pyrimidine De Novo Synthesis: Step 4
Make UDP/UTP
- revsible rxns
- carried out by kinases (that add P)

Pyrimidine De Novo Synthesis: Step 5
Convert UTP → CTP
- catalyzed by CTP synthetase: transaminates C= O → NH2 (donated from GLUTAMINE)
- inhibited by CTP (feedback inhibition)
- UTP & CTP make RNA

Pyrimidine De Novo Synthesis: Step 6
CDP → dCDP
- ribonucleotide reductase = deoxygenates
- Clinical Correlation:*
HYDROXYUREA = a drug that inhibits reductase so no dCDP is made

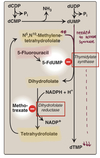
Thymidine
- Only Used in DNA
- Deoxythymidine is only required nucleotide
- Synthesized from deoxyuridine
Thymidine Synthesis: Step 1
Convert UMP → dUDP
- INVOLVES RIBONUCLEOTIDE REDUCASE

Thymidine Synthesis: Step 2 & 3
Convert dUD**P → dU**MP (via phorphorylase)
Convert dU**MP → d**TMP (via thymidylate synthase)
- thymidylate synthase requires THF to be the methyl donor
- methotrexate inhibits dihydrofolate reductase ( = NO N5N10-MethyleneTHF)
- 5-FdUMP inhibits dihydrofolate thymidylate synthase ( NO dihydrofolate = NO N5N10-MethyleneTHF)

5-FdUMP
5-Fluorouracil (S phase)
DRUG: chemotherapy
- mimics uracil strcuture
-
INHIBITS Thymidylate synthase = ↓↓↓ dTMP (“thymineless death”)
- permenant covalent bonding = inhibition
Enzyme normal function:
Methylates dUMP to dTMP requires THF

Hydroxyurea (S phase)
DRUG
- INHIBITS Ribonucleotide reductase
Normal function of enzyme:
Reduces all NDPs to dNDPs for DNA synthesis

Methotrexate (eukaryotic S phase)
DRUG = chemotherapy/ immunosuppressant
- INHIBITS Dihydrofolate reductase (DHFR) = ↓ Thymidine
- mimics DHF
normal function of enzyme:
- Converts DiHydroFolate to TetraHydroFolate
- Without DHFR, thymidylate synthesis will eventually stop
RESCUE (chemoprotectant):
Leucovorin : another way of making THF
Vitamin B12
FOLATE
- essential for thymidine production*
- ALLOWS RECYLING of THF
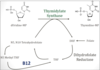
Folate Deficiency
- ↓↓↓ dTMP production = ↓↓↓ DNA production
- RNA is okay b/c it does not need T
Leads to:
- Macrocytic Anemia ( fewer but larger RBC)
- Neural Tube Defects in pregnancy
Vitamin B12 Deficiency
- needed to recycle THF from N5-Methyl-THF
- deficiency = “Methyl Folate Trap”
- Megaloblastic Anemia: NO dTMP synthesis
- Demyelination: neurological disfunction
Homocysteine and MethylMalonic Acid
Homocysteine
- require BOTH Folate & B12 to produce methionine
- therefore, you will see ↑↑↑ homocysteine in BOTH deficiencies (↓↓↓)
Methyl Malonic Acid
- only B12 to convert MMA → succinyl CoA
- therefore, ↑↑↑ MMA in B12 Deficiency (B12 ↓↓↓)
- folate deciciency = normal MMA

Pyrimidine Salvage Synthesis
- Pyrimidine synthesis is COSTLY (requires 5 mole ATP for 1 mol UMP)
- nucleosides salvagable
- primary enzymes = nucleoside kinases
Pyrimidine Catabolism
- De-phosphorylation
- phosphoroLYSIS
- De-Amination
AA needed for Purine Synthesis
- Glycine
- Aspartate
- Glutamine
First commited step in Purine Synthesis
PRPP → phosphoribosylamine

First Compound with Complete Purine Ring
IMP

Purine De Novo Synthesis: Step 1
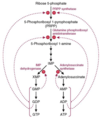
Step 1: Create PRPP
- starting with ribose-5-phosphate (from PPP):
- anomeric carbon of ribose (C-1’) serves as foundation
- R5P → PRPP catalyzed by 5’- PhosphoRibosyl-1-PyroPhosphate (PRPP) synthetase
- Since, PRPP used elsewhere, it is NOT Commited Step

Purine De Novo Synthesis: Step 2
(High Yield)
Step 2: Displacement of Pyrophosphate by N Group in Glutamine
- catalyzed by amidophospho ribosyltransferase OR Glutamine phosphoribosyl amido transferase
- committed & rate-limiting step : amide N of Glutamine → N-9 in purine ring

Purine De Novo Synthesis: Step 3 - 6
Step 3 to 6: Synthesis of IMP
requires:
- Glycine
- Tetrahydro folate
- Glutamine
- CO2
- Aspartate

Branch Point Synthesis
Ribavarin & Mycophenolate INHIBIT IMP DEHYDROGENASE = NO GMP

Deoxygenation of ADP & GDP
- via Ribonucleotide reductase

Purine Pathway Regulation
- feedback regulation of PRPP synthetase, ATase, and both branch point pathways
- (cross-regulation): stimulation of branch point synthesis by end-product of opposite branch
- ATP stimulates IMP → GMP (ie ↑ ATP = ↑ GMP)
- GTP stimulates IMP → AMP (ie ↑ GTP = ↑ AMP)
Purine Fates & Salvage
Salvages bases: adenine, guanine, hypoxanthine
Converts back into nucleotides: AMP, GMP, IMP
Requires PRPP

Catabolism Step 1: dephosphorylation
Step 1 of Catabolism
-
phosphate removed: monophosphate → respective nucleoside
- catalyzed by family of 5’-nucleotidases (5’-NT)
- once relieved of charged phosphate, resulting nucleoside can readily cross cell membrane
Catabolism Step 3: deamination
- amino group removed from _C-6 of A_denine or _C-2 of G_uanine
- catalyzed by AMP deaminase, adenosine deaminase (ADA), or guanase

Catabolism Step 2: phosphorolysis
- pentose sugar removed: converts purine nucleoside → respective base
- catalyzed by purine nucleoside phosphorylase (PNP)
AMP catabolism is differentially regulated per tissue…
-
heart: produces adenosine (diffusable)
- coronary vasodilator: facilitates oxygen delivery to damaged tissue
- skeletal muscle: produces IMP (NOT diffusible)
- facilitates net resynthesis of ATP following exercise
AMP & GMP catabolism = ↑↑↑ of hypoxanthine and xanthine
- AMP → hypoxanthine
- GMP → xanthine
- these bases oxidized to uric acid (urate) by xanthine dehydrogenase (XDH)
- uses NAD+ as electron acceptor (preferentially)
- XDH → XO
Adenosine Deaminase (ADA) Deficiency
-
results in SCID: severe combined immunodeficiency
- both T-cell & B-cell dysfunction
- dATP = potent RR inhibitor
- In ADA deficiency, dATP accumulates


