Module 4.1 - Basic Concepts and Hydrocarbons Flashcards
(81 cards)
What is nomenclature?
The system of naming compounds
What is a homologous series?
A series of organic compounds that have the same functional group with each successive member differing by CH2
What is an aliphatic hydrocarbon?
A hydrocarbon with carbon atoms joined together in straight or branched chains
What is an alicyclic hydrocarbon?
A hydrocarbon with carbon atoms joined together in a ring structure (not aromatic)
What is an aromatic hydrocarbon?
Contains at least one benzene ring
Are alkanes saturated or unsaturated?
Saturated
What is the first 10 compounds of the alkane homologous series and what is their formulae?
- methane, CH4
- ethane, C2H6
- propane, C3H8
- butane, C4H10
- pentane, C5H12
- hexane, C6H14
- heptane, C7H16
- octane, C8H18
- nonane, C9H22
- decane, C10H22
What are the first 10 alkyl groups and their formulae?
- methyl, CH3
- ethyl, C2H5
- propyl, C3H7
- butyl, C4H9
- pentyl, C5H11
- hexyl, C6H13
- heptyl, C7H15
- octyl, C8H17
- nonyl, C9H19
- decyl, C10H21
What is the formula, prefix and suffix of the alcohol functional group?
- OH
prefix: hydroxy-
suffix: -ol
What is the formula and suffix of the aldehyde functional group?
- CHO
- al

What is the suffix and formula of the alkane functional group?
C-C
-ane
What is the suffix and formula of the alkene functional group?
C=C
-ene
What is the formula and suffix of the carboxylic acid functional group?
- COOH
- oic acid

What is the formula and prefix for the haloalkane funcitonal group?
- F, fluoro-
- Cl, chloro-
- Br, bromo-
- I, iodo-
What is the formula and suffix of the ketone functional group?
C-CO-C
-one

What is a saturated compound?
Compounds that have only single bonds
What is a functional group?
A group of atoms that is responsible for the characteristic chemical reactions of a compound
What is the general formula of alkanes?
CnH2n+2
What is the general formula of alkenes?
CnH2n
What is the general formula of alcohols?
CnH2n+1OH
What is a general formula?
The simplest algebraic formula for a homologous series
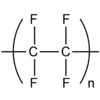
What is a displayed formulae?
Shows the relative positions of atoms and the bonds between them
What is a structural formula?
Provides the minimum detail for the arrangement of atoms in a molecule
What is an empirical formula?
Shows the smallest whole number ratio of atoms of the elements in a compound