Microbiology 2: Antimicrobials 1 Flashcards
What are some targets of antimicrobials?
- Peptidoglycan layer of cell wall
- Inhibition of bacterial protein synthesis
- DNA gyrase and other prokaryote specific enzymes
What are some antibiotic classes that inhibit peptidoglycan synthesis
beta-lactam antibiotics, glycopeptides
Give some examples of Beta lactam antibiotics
penicillins, cephalosporins, carbapenems
Give some examples of Glycopeptides antibiotics
vancomycin, teicoplanin
Do Beta lactam antibiotics target gram positive or gram negative bacteria?
broad spectrum
Do Glycopeptides antibiotics target gram positive or gram negative bacteria?
- Gram-positive
What is the difference between gram positive and gram negative bacteria?
- Gram-positive cell wall = thick peptidoglycan cell wall (made of NAG and NAM components)
- Gram-negative cell wall = thinner peptidoglycan cell wall, outer membrane conferring resistance to some antibiotics
- Can be more resistant and harder to treat due to outer membrane
What is beta lactam antibiotics’ mechanism of action?
-
Inactivate enzymes involved in terminal stages of cell wall synthesis = transpeptidases / penicillin binding proteins
- Beta lactam is a structural analogue of the enzyme substrate
-
Bactericidal (active against rapidly dividing bacteria) – if cell wall has already been formed, they have no effect
- Ineffective against bacteria lacking peptidoglycan cell walls (mycoplasma, chlamydia)
- Cause cell lysis

What type (gram positive or gram negative) of bacteria does penicillin target? Give some examples
- gram +ve,
- streptococci, clostridia
What is penicillin broken down by?
- beta-lactamase
- produced by S. aureus (SA) and many other gram -ve organisms
What type (gram positive or gram negative) of bacteria does amoxicillin target? Give some examples
- broad-spectrum
- (enterococci to gram -ve)
What is amoxicillin broken down by?
- beta-lactamase
- produced by S. aureus (SA) and many other gram -ve organisms
What type (gram positive or gram negative) of bacteria does flucloxacillin target? Give some examples
- gram negative, ONLY S. aureus
What is flucloxacillin broken down by?
- Not broken down by beta-lactamase produced by SA
- used to treat SA infections (S. aureus)
Compare flucloxacillin and penicillin
- Similar to penicillin, less reactive
What type (gram positive or gram negative) of bacteria does Piperacillin target? Give some examples
- broad-spectrum
- (pseudomonas, non-enteric gram -ve)
What is Piperacillin broken down by?
- Broken down by beta lactamase
- (produced by SA and many other gram -ve organisms)
Which antibiotic is Piperacillin similar to?
amoxicillin
What is the antibiotic name for Clavulanic acid
Co-amoxiclav
What is the antibiotic name for tazobactam?
Tazocin / Piptazobactam
What is clavulanic acid and how does it work?
beta lactamase inhibitors –> protect penicillin from enzymatic breakdown
What is the point of combining Clavulanic acid (Co-amoxiclav) and tazobactam (Tazocin / Piptazobactam)?
- Inhibit beta lactamase from being broken down by bacteria (protect penicillins from breaking down)
- Increase coverage to include SA, gram -ve (i.e. E. coli), anaerobes
Which organisms are resistant to cephalosporins? What should be used instead
ESBL producing organisms resistant to cephalosporins –> use carbapenems
Give some examples of cephalosporins antibiotics
- Cefuroxime
- Ceftriaxone
- Ceftazidime
What is Cefuroxime broken down by?
- Stable to many beta lactamases made by gram -ve
Compare co-amox and Cefuroxime
- Similar cover to co-amox (less active against anaerobes)
- if anaerobes suspected, add metronidozole to cefuroxime
What is ceftriaxone associated with?
- C. difficile
What is ceftriaxone used to treat?
- Treat meningitis (IM ceftriaxone)
Which organism does ceftriaxone NOT cover against?
- NO COVER against Pseudomonas
Which organisms does ceftazidime provide cover against?
- Activity against pseudomonas (HAIs often)
What is the advantage of using Ceftazidime over Ceftriaxone?
- Ceftazidime = activity against pseudomonas (HAIs often)
- Ceftriaxone = no activity against pseudomonas
Which type of patients is Cefotaxime used to treat?
- Cefotaxime = the paediatric ceftriaxone
Are ESBL producing organisms resistant to carbapenems?
ESBL producing organisms NOT resistant to carbapenems
Give some examples of carbapenem antibiotics
- Meropenem, imipenem, ertapenem
Why do MDR organisms pose a threat to carbapenem use?
production of carbapenemase enzymes becoming more widespread
Which bacterial species are becoming more multi-drug resistant (MDR)?
Acinetobacter and klebsiella species
Give an example of a Monobactam antibiotic
- Carumonam
What are the key features of beta lactam antibiotics?
- Relatively non-toxic
- Renally excreted so decrease dose if renal impairment
- Short T1/2 (many are type 2 drugs so aim to maximise the time > MIC)
- Will not cross BBB
- Cross allergenic – penicillin has 10% cross reactivity with cephalosporins and carbapenems
Do Glycopeptides antibiotics target gram positive or gram negative bacteria?
gram +
What is the mechanism of action for glycopeptide antibiotics?
inhibit cell wall synthesis (hence target gram +tive)

Why are glycopeptide antibiotics unable to target gram -tive bacteria?
- Large molecules so unable to penetrate gram -ve
What are the important uses of glycopeptide antibiotics?
- MRSA infections (IV)
- C. difficile infection (oral – Vancomycin, teicoplanin)
What is the major complication/risk of using glycopeptide antibiotics?
- Nephrotoxic – must monitor for accumulation
Which antibiotic classes are inhibitors of Protein Synthesis?
- Aminoglycosides
- Tetracyclines
- Macrolides
- Chloramphenicol
- Oxazolidinones
Give some examples of Aminoglycoside antibiotics
gentamicin, amikacin, tobramycin
**Give some examples of tetracycline antibiotics
??
Give some examples of macrolide antibiotics
erythromycin, lincosamides – clindamycin, streptogramins – synercid – MLS group
Describe the mechanism of action of Aminoglycoside antibiotics
- Bind to amino-acyl site of 30s ribosome subunit
- Rapid, concentration-dependent bactericidal
- Require specific transport mechanisms to enter
- Accounts for some intrinsic resistance

What are some possible complications of aminoglycoside antibiotics?
- Ototoxic and nephrotoxic – monitor levels
Which of the 3 aminoglycoside antibiotics are particularly active against pseudomonas aeruginosa?
Gentamicin and tobramycin
When combined with beta lactams, what can aminoglycosides be used to treat?
-
Synergistic combinaton with beta lactams
- Endocarditis treatment, pneumonia
Do aminoglycosides have any activity against aenerobes?
no
What type (gram positive or gram negative) of bacteria does Tetracyclines target?
Broad spectrum
What kind of organisms (intracellular or extracellular) do tetracyclines target? Gice some examples
- activity against intracellular pathogens –
- chlamydia, rickettsia, mycoplasma
What is the mechanism of action of tetracyclines?
- Bacteriostatic (stops bacteria from reproducing)

What are the problems with using tetracyclines?
- Widespread resistance now
- Deposited in growing bone
- Don’t give to children (<12 yrs), pregnant women
- SE: photosensitivity rash (summer effect)
What is the mechanim of action of macrolides?
- Bacteriostatic (stops bacteria from reproducing)

What are the benefits of using macrolides?
- Useful agent for treating mild staphylococcal or streptococcal infections in pen-allergic patients
- Active against campylobacter species, legionella, pneumophilia
What are some newer macrolide agents, and why are they useful?
- Newer agents include clarithromycin and azithromycin
- due to a better half-life
Are macrolides useful against gram -tive bacteria?
- Little activity against gram -ve bacteria (membrane)
What type (gram positive or gram negative) of bacteria does chloramphenicol target?
broad spectrum
What is the mechanism of action of chloramphenicol?
- Bacteriostatic

How often is chloramphenicol used?
What are the risks/complications of its/their use?
-
Rarely used apart from eye preparations
- Risk of aplastic anaemia
- Risk of grey-baby syndrome in neonates because of inability to metabolise the drug
What is the main antibiotic in the Oxazolidinone class?
Linezolid
What type (gram positive or gram negative) of bacteria does Oxazolidinones target?
- Highly active against gram +ve (MRSA & VRE)
- Not active against most gram -ve
What are the downsides of Oxazolidinones?
- Expensive,
- may cause thrombocytopenia & optic neuritis;
- should only be used with micro/ID approval
What is the mechanism of action of Oxazolidinones ?
- Binds to 23S component of 50s subunit à prevents formation of a functional 70s initiation complex
Name the antibiotic classes that are inhibitors of DNA synthesis
- Quinolones / Fluoroquinolones
- Nitroimidazoles
Name some examples of Fluoroquinolones
- ciprofloxacin (old),
- levofloxacin (new),
- moxifloxacin (new)
What type (gram positive or gram negative) of bacteria do Fluoroquinolones target?
- Broad antibacterial activity versus gram -ve (pseudomonas aeruginosa)
What is the mechanism of action of Fluoroquinolones
- Act on alpha unit of DNA gyrase, bactericidal
What are the newer agents of fluroquinolones? Which organisms are they better against?
- Newer agents (levofloxacin, moxifloxin)
- better against gram +ve and intracellular bacteria (Chlamydia spp.)
What are Fluoroquinolones used to treat?
- UTI,
- pneumonia,
- atypical pneumonia,
- bacterial gastroenteritis
Name some examples of Nitroimidazoles
- metronidazole,
- tinidazole
What is the mechanism of action of Nitroimidazoles?
- Under anaerobic conditions, an active intermediate is produced which causes DNA strand breakage
- Rapidly bactericidal
Which organisms are Nitroimidazoles active against?
- Active against anaerobic bacteria and protozoa (Giardia)
What are nitrofurans? What are they used to treat?
- Nitrofurans are related compounds to Nitroimidazoles
- nitrofurantoin is good for cystitis and lower UTIs – take after voiding bladder
Which classes of antibiotics are inhibitors of RNA Synthesis?
Rifamycins
Name some examples of Rifamycins
- rifampicin
- rifabutin
What is the mechanism of action of Rifamycins?
- Inhibits protein synthesis by binding to DNA-dependent RNA polymerase, inhibiting initiation
- Bactericidal
Which organisms are Rifamycins active against?
- mycobacteria
- chlamydia
Where is rifamycin metabolised? And hence which bloods must be done regularly?
- Interactions with other drugs metabolised in the liver (e.g. OCP)
- and so need to monitor LFTs
How might you check compliance with Rifamycins?
- Turns secretions orange (urine and contacts) – can check compliance
Which of the Rifamycin antibiotics have bacteria developed resistance to? And how?
-
Rifampicin resistance (never used as a single):
- Resistance due to chromosomal mutation
- Causes single amino acid change in beta subunit of RNA polymerase which fails to bind rifampicin
Which classess of antibiotics act by releasing Cell Membrane Toxins?
- Daptomycin
- Colistin
What type (gram positive or gram negative) of bacteria does Daptomycin target?
activity limited to gram +ve
What is the molecular structure of Daptomycin?
Cyclic lipopeptide
What can Daptomycin be used to treat? When may this be necessary?
- MRSA and VRE infections
- as an alternative to linezolid and synercid (e.g. if patient is intolerant)
How is Colistin administered?
IM/IV
What is the structure of Colistin?
Polymyxin antibiotic
What type (gram positive or gram negative) of bacteria does Colistin target? Give some examples
- Active against gram -ve including:
- pseudomonas aeruginosa, Acinetobacter baumannii, klebsiella pneumoniae
What are some possible complications of Colistin use? Hence, what is it reserved for?
- Nephrotoxic
- reserved for use against multi-resistant organisms
Which classes of antibiotics are Inhibitors of Folate Metabolism?
- Sulphonamides
- Diaminopyrimidines
How do antibiotics that are Inhibitors of Folate Metabolism work?
These act indirectly on DNA through interference with folic acid metabolism
Give an example of synergistic action between 2 drug classes
(Synergistic action between 2 drug classes because they act on sequential stages in the same pathway)
- I.E. co-trimoxazole = sulphamethoxazole + trimethoprim
What is the problem with Sulphonamides
Resistance is common
What combination is important in treating pneumocystis jiroveci pneumonia? (PCP – HIV-defining disease)
Combination of sulphamethoxazole + trimethoprim (co-trimoxazole)
Give an example of a Diaminopyrimidines
trimethoprim
What is trimethoprim (Diaminopyrimidine) used to treat?
Used as treatment for community acquired UTIs
What is the acronym for methods of antibacterial resistance?
BEAT

Which antibiotic classes are susceptible to inactivation?
- beta lactams
- aminoglycosides
- chloramphenicol
Which antibiotic classes are susceptible to altered targets (method of antibiotic resistance)?

Which antibiotic classes are susceptible to reduced accumulation (of the antibiotic)?
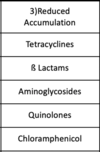
Which antibiotic classes are susceptible to bypass antibiotic-sensitive step (method of antibiotic resistance)?
- trimethoprim
- sulphonamides
Which organisms produced beta lactamases as a major mechanism of resistance to beta lactam ABx?
SA and gram -ve bacilli (coliforms)
Which organisms are not penicillin resistant?
- group A (strep pyogenes), B, C, or G beta haemolytic streptococci
What is MRSA’s method of antibiotic (methicillin) resistance?
- altered target
- mecA gene encodes novel penicillin binding protein (PBP)(2A) / novel PBP 2a
- Low affinity for binding beta lactams
- Substitutes for essential functions of high affinity PBPs at otherwise lethal concentrations of antibiotics
How is Streptococcus pneumoniae resistant to penicillin?
- Penicillin resistance is the result of acquisition of stepwise mutations in PBP genes
- Lower level resistance can be overcome by increasing dose of penicillin used
How is resistance to macrolides acquired by bacteria?
- Adenine-N6 methyltransferase modifies 23S rRNA à reduces binding of MLS antibiotics and results in resistance
- Encoded by erm (erythromycin ribosome methylation) genes.
What is ESBL-based Resistance?
Extended spectrum beta lactamases
Which antibiotic classes can ESBLs break down?
-
cephalosporins (cefotaxime, ceftazidime, cefuroxime) as well as penicillins
- But, not carbapenems
Which organisms produce ESBLs?
- More common in E. coli and Klebsiella
What are new beta lactamases spreading?
New beta-lactamases are spreading MDR instead of just the ESBL-component of resistance


