L17- Liver function (Biochem) Flashcards
(20 cards)
Functions of the liver
- Carbohydrate metabolism
- Amino acid metabolism
- Lipid metabolism
- Haem degradation
Liver fructolysis
- In normal tissues, fructose is degraded to F6P via hexokinase, which will then be converted to G6P by phosphohexose isomerase, and enter glycolysis pathway. However the initial conversion by hexokinase is slow as hexokinase has a lower affinity for fructose than for glucose. Metabolism for fructose increases only when fructose accumulates.
- in liver, however, the major enzyme metabolizing fructose would be fructokinase, which will convert fructose to F1P
- F1P will then be broken down into DAP (dihydroxyacetone phosphate) & glyceraldehyde by F1P aldolase
- Dihydroxyacetone phosphate is converted to glyceraldehyde 3-phosphate by the glycolytic enzyme triose phosphate isomerase. Glyceraldehyde is phosphorylated by ATP and triose kinase to glyceraldehyde 3-phosphate
- G3P then enters glycolytic pathway or gluconeogenesis pathway

Liver glycolysis
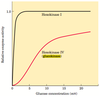
Main glycolysis pathway of hepatocytes retain the same enzymes and pathways as do normal cells. But a different isozyme - glucokinase - is involved.
The glucose transporter in hepatocytes (GLUT2) is so effective that the concentration of glucose within a hepatocyte is essentially the same as that in the blood. Glucose entering hepatocytes is phosphorylated by hexokinase IV (glucokinase) to yield glucose 6-phosphate.
Glucokinase has a much higher Km for glucose (10 mM) than do the hexokinase isozymes in other cells and, unlike these other isozymes, it is not inhibited by its product, glucose 6-phosphate.
The presence of glucokinase allows hepatocytes to continue phosphorylating glucose when the glucose concentration rises well above levels (e.g. after meal) that would overwhelm other hexokinases. The high Km of glucokinase also ensures that the phosphorylation of glucose in hepatocytes is minimal when the glucose concentration is low (e.g. fasting), preventing the liver from consuming glucose as fuel via glycolysis (i.e. glucokinase only show greater activity when glucose level increases)
Note: glucokinase exists in liver, pancreas, hypothalamus and small intestine as well
Liver gluconeogenesis
Hepatic glycogen is broken down to G-6P. G-6 phosphatase exists in liver to convert G-6P back to glucose, which will then be released to the bloodstream in fasting state. The glucose produced will be used for maintenance of blood glucose level.
(sidenote: G-6 phosphatase also exists in kidneys’ cortical cells, which helps to produce glucose to support medullary cells)
Liver galactose metabolism
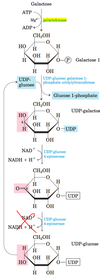
Galactose, a product of hydrolysis of the lactose, passes in the blood from the intestine to the liver, where it is first phosphorylated by the enzyme galactokinase to form galactose 1-phosphate
Galactose 1-phosphate will then displace glucose 1-phosphate from UDP-glucose (via Gal-1-P UDPG transferase) forming UDP- galactose, and releasing glucose 1-phosphate. UDP-galactose is then converted to UDP- glucose (via UDP-Gal UDPG isomerase), which is recycled through another round of the same reaction.
The net effect of this cycle is the conversion of galactose 1-phosphate to glucose 1-phosphate, which will be converted to glucose 6- phosphate and enter glycolysis or gluconeogenesis; there is no net production or consumption of UDP-galactose or UDP-glucose.
Fed state liver protein metabolism
Fast state liver protein metabolism
Transdeamination of amino acid in liver
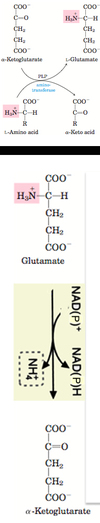
In liver cells, transamination is coupled to oxidative deamination to release the amino group as ammonia. Transamination takes place in the cytoplasm while oxidative deamination occurs in mitochondria.
1) In the presence of enzyme aminotransferase (e.g. Aspartate aminotransferase/ Glutamate- oxaloacetate transaminase) and the cofactor pyridoxal phosphate (PLP), amino group in amino acids (e.g. aspartate) are removed from amino acids, leading to formation of α-keto acids (e.g. oxaloacetate). The amino group is usually transported to α-ketoglutarate, forming glutamate.
2) The amino group-containing glutamate then enters the mitochondria, and is converted to α-ketoglutarate via glutamate dehydrogenase, liberating ammonia
Sources of NH4+
Hyperammonemia
A failure of the liver to dispose excess ammonia derived from catabolism of N-containing compounds.
The excessive ammonia may enter brain cell and lead to damage by the following ways:
1) Ammonia may combine with α-ketoglutarate to form glutamate
2) The glutamate might acquire another ammonium group to form glutamine and export away by blood
3) As a result, since the TCA cycle intermediate α-ketoglutarate is greatly removed, leading to energy depletion and damage
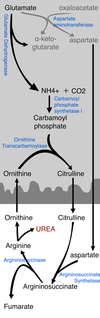
Urea Cycle
One amino group enters the urea cycle as carbamoyl phosphate, formed in the matrix; the other enters as aspartate, formed in the matrix by transamination of oxaloacetate and glutamate, catalyzed by aspartate aminotransferase.
1) NH4+, along with CO2, is first converted to Carbamoyl Phosphate in the mitochondrion matrix, by carbamoyl phosphate synthase I (CPS I)
2) Carbamoyl Phosphate combines with Ornithine to form Citrulline in the mitochondrion matrix, via ornithine transcarbamoylase; the citrulline then passes into the cytosol
3) Citrulline combines with Aspartate to form Argininosuccinate, through argininosuccinate synthetase.
4) Argininosuccinate is converted to Arginine and fumarate, through argininosuccinase. Fumarate will then enter the citric acid cycle.
5) Arginine forms urea and regenerates ornithine, which then re-enters mitochondrion matrix to react with carbamoyl phosphate
Feed-forward regulation
The first enzyme in the urea formation pathway, carbamoyl phosphate synthetase I, is allosterically activated by N-acetylglutamate, which is synthesized from acetyl- CoA and glutamate by N-acetylglutamate synthase.
In mammals N-acetylglutamate synthase activity in the liver has a purely regulatory function.
The steady-state levels of N-acetylglutamate are determined by the concentrations of glutamate and acetyl-CoA (the substrates for N-acetylglutamate synthase) and arginine (an activator of N-acetylglutamate synthase, and thus an activator of the urea cycle)

Why is ornithine not used to make proteins?
Ornithine, while an amino acid, is not used to make protein in the human boy because there is no codon encoding for ornithine in the human genome
Glucose -Alanine cycle
Alanine plays a special role in transporting amino groups to the liver in a nontoxic form, via the glucose-alanine cycle:
1) In muscle and certain other tissues that degrade amino acids for fuel, amino groups are collected in the form of glutamate by transamination
2) Glutamate can be converted to glutamine for transport to the liver; or it can transfer its α-amino group to pyruvate, forming α-ketoglutarate and alanine, by the action of alanine aminotransferase
3) Alanine passes through blood into the liver
4) In the cytosol of hepatocytes, alanine aminotransferase transfers the amino group from alanine to α-ketoglutarate, forming pyruvate and glutamate.
5) Pyruvate will undergo gluconeogenesis to form Glucose. Glucose will go through blood to re-enter tissues and undergo glycolysis.
6) Glutamate can then enter mitochondria, where it can undergo deamination to release NH4+ via glutamate dehydrogenase reaction; or can undergo transamination with oxaloacetate via aspartate aminotransferase to form α-ketoglutarate and aspartate.
6) Aspartate and NH4+ both donate nitrogen to form urea which is then excreted

Ketogenesis in Liver
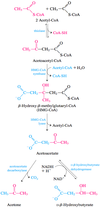
Ketone bodies generated are acetoacetate, acetone and D-ß- hydroxybutyrate
1) Fatty acids is formed from TG in adipose tissue; FA enters mitochondria
2) Fatty acids forms acetyl CoA via ß oxidation
3) 2 Acetyl CoA is converted to acetoacetyl CoA via thiolase
4) Acetoacetyl CoA is converted to HMG-CoA via HMG CoA Synthase
5) HMG-CoA is converted to acetyl CoA and Acetoacetate via HMG CoA Lyase
6) Acetoacetate can then be converted to acetone via acetoacetate decarboxylase; or to D-ß- hydroxybutyrate via D-ß- hydroxybutyrate dehydrogenase
Regulation of ketogenesis
In case when lipolysis is increased (e.g. fasting, high fat low carobohydrate diet, or uncontrolled diabetes), glucagon-insulin ratio will increase, leading to increased ketogenesis
[Starvation and untreated diabetes mellitus lead to overproduction of ketone bodies, with several associated medical problems.
During starvation, gluconeogenesis depletes citric acid cycle intermediates, diverting acetyl-CoA to ketone body production.
In untreated diabetes, when the insulin level is insufficient, extrahepatic tissues cannot take up glucose efficiently from the blood, either for fuel or for conversion to fat. Under these conditions, and fatty acids enter mitochondria to be degraded to acetyl- CoA — which cannot pass through the citric acid cycle because cycle intermediates have been drawn off for use as substrates in gluconeogenesis. The resulting accumulation of acetyl-CoA accelerates the formation of ketone bodies beyond the capacity of extrahepatic tissues to oxidize them.
The increased blood levels of acetoacetate and D-ß- hydroxybutyrate lower the blood pH, causing the condition known as acidosis. Extreme acidosis can lead to coma and in some cases death. Ketone bodies in the blood and urine of untreated diabetics can reach extraordinary levels - this condition is called ketosis.]

Oxidation of
ketone bodies
Acetone, produced in smaller quantities than the other ketone bodies, is exhaled.
Acetoacetate and D-ß- hydroxybutyrate are transported by the blood to extrahepatic tissues (skeletal muscles, heart muscles, renal cortex, brain), and they are converted to acetyl-CoA and oxidized in the TCA cycle, providing energy. These are more common under starvation conditions, when glucose is unavailable.
[in prolonged fasting, muscles switch from dependence on oxidation of ketone bodies to that of fatty acid as source of energy]
The liver does not actively utilise ketone bodies as thiotransferase activity is low in hepatocyte mitochondria
1) D-ß- hydroxybutyrate is converted to acetoacetate via D-ß- hydroxybutyrate dehydrogenase
2) acetoacetate react with succinyl CoA to form Acetoacetyl- CoA via thiotransferase (?)
3) acetoacetyl- CoA is converted to 2 acetyl- CoA by thiolase; acetyl-Co A will enter TCA cycle

Heme degradation

Location of Conversion of haem to bilirubin
In reticuloendothelial system of the spleen

Formation of Bilirubin diglucuronide
The conjugation reaction is catalyzed by UDP-glucuronosyl transferase in ER of hepatocytes



