Free radical substitution and nucleophilic substitution Flashcards
define nucleophile
an e- pair donor
contains an area of high e- density (typically negatively charged)
define free radical
an atom or group of atoms which have a single unpaired electron (shown as a single black dot next to the atom to which it belongs)
occurs when due to homolytic fission (when a bond pair of e- is split evenly and each of the atoms gets an e-)

what is the main use of free radical substitution?
to make haloalkanes-one or more of the H atoms are substituted by a halogen atom
name and outline the mechanism for this reaction including each step
CH4 + Cl2 → CH3Cl + HCl
this is an example of free radical substitution
First step: initiation
Cl2 → 2Cl• (in the presence of UV light)
Step 2: propagation
the chlorine free radical steals a H atom from the alkane
a dot is placed above the C which has lost a H atom
Cl• + CH4 → •CH3+ HCl
the newly formed free radical then reacts with Cl2 to form the haloalkane ( a Cl atom is stolen )
•CH3 + Cl2 → CH3Cl + Cl•
This reforms the chlorine free radicals which can react with the other methane molecules and so on, resulting in a chain reaction
Step 3: Termination
when the free radicals combine, the reaction terminates
2Cl• → Cl2
2•CH3 → CH3CH3
Cl• + •CH3 → CH3Cl (chloromethane also formed here)
Write the free radical substitution equations which occur in the ozone layer and describe why this process is a problem
Cl• + O3 ⟶ ClO• + O<span>2</span>
ClO• + O3 ⟶ 2O2 + Cl•
Ozone (O3) is an allotrope of oxygen found in the stratosphere where it absorbs some of the harmful UV radiation from the sun. UV light can damage plant tissue, reduce plankton populations in the ocean and cause skin cancer
Ozone levels have been decreasing due to the free radical substitution reactions with halogen free radicals which result in its break down to O2
Cl free radicals are the most dangerous. They are found in CFCs which are used as fire extinguishers and refrigerants. When CFCs enter the stratosphere, the UV light results in the homolysis of the C-Cl bond releasing the Cl•
why do unsaturated compound not react with nucleophile
the -C=C- bond is an are of high e- density so repels the nucleophile (as it has a negative charge)
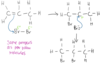
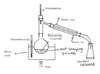
draw a diagram of reflux apparatus
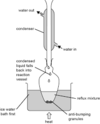
draw a diagram of the apparatus needed for simple distillation
Outline the mechanism and state the type of reaction and the conditions needed for the reaction between a haloalkane and KOH
conditions:
- Dissolve haloalkane in a small volume of ethanol
- Add aqueous solution of NaOH (or any other hydroxide ion containing compound)
- Warm reflux
type of reaction = hydrolysis

Outline the mechanism and state the conditions needed for the reaction between a haloalkane and KCN
conditions
- Dissolve haloalkane in a small volume of ethanol
- Add aqueous solution of KCN (or any other cyanide ion containing compound)
- Warm reflux

Outline the mechanism and state the conditions needed for the reaction between a haloalkane and NH3
conditions:
- Dissolve haloalkane in a small volume of ethanol
- Add excess concentrated ammonia solution
- Seal container under high pressure

How would you increase the likelihood that a primary amine is formed from the nucleophilic substitution of NH3 into a haloalkane
- to prevent primary and secondary amines from act as nucleophiles to react with the the haloalkane and result in further substitution, add excess NH3
- this means it is more likely for a :NH3 nucleophile to react with the haloalkane
define reflux and explain why its useful
- continuous boiling and condensing of a reaction mixture
- helps increase the rate of reaction and yield of products as nucleophilic reactions are too slow at room temp
expalin why the flask in reflux apparatus is not sealed and why anti-bumping granules are added
- not sealing the flask prevents the build up of pressure inside the apparatus
- anti-bumping granules ensure smooth boiling
explain why haloalkanes are able to partake in nucleophilic substitution reactions
- C delta + of polar C-X bond is susceptible to nucleophilic attack
explain how you would compare the reactivity of different haloalkanes using hydrolysis reactions
- Add a known amount of haloalkane and the same amount of AgNO3 solution in a mixture of ethanol and water. Keep the temp the same
- Measure the time taken for a specific amount of precipitate to form (or draw a black X on test tube and measure time taken for X to disappear)
- Calculate rate using amount of ppt/time or 1/time
- The ethanol and water form hydroxide ion nucleophiles which go on to react with the haloalkane to form an alcohol and halide ion leaving groups
- The precipitate forms when the halide ion has been released from the haloalkane
- The weaker the C-X bond in the haloalkane, the easier it is to release the halide ions, so the faster the formation of the precipitate=higher RoR=higher reactivity
what is the general formula for haloalkanes?
CnH2n+1X
outline the mechanism and state the conditions for the elimination reaction of a haloalkane
conditions:
- KOH (or any other OH containing compound) is dissolved in ethanol
- Anhydrous
- Hot reflux
what happens:
role of :OH nucleophile = act as a base (H+ acceptor)
Step 1) A H+ is removed from the C atom next to the C-X bond
Step 2) A C=C bond forms between the C next to the C-X and the C in the C-X bond
Step 3) A H2O molecule and a halide ion leaving group are formed

state which mechanism is more likely to occur between a :OH- nucleophile and a 1º, 2º or 3º haloalkane
1º haloalkane = substitution more likely
2º haloalkane = equally likely for either substitution or elimination
3º = elimination more likely
define electrophile
- electron pair acceptor
- They are electron deficient species so are attracted by areas of high electron density
- Often polar molecules
why do alkenes undergo electrophilic addition?
the C=C bond is an area of high e- density so is susceptible to electrophilic attack
define heterolytic fission and give two examples where it happens
- when the covalent bond is broken unevenly so one atom receives both e- while the other receives none
- this produces a positive ion and a negative ion
- nucleophilic substitution and electrophilic addition involve heterolytic fissions
Outline the mechanism between an alkene and HBr

outline the mechanism between an alkene and Br2
When the neutral molecule approaches the C=C bond, a temporary dipole is induced because the high e- density repels the electrons in the neutral molecule more towards one of the Br atoms
no major/minor products as product is always the same no matter which carbocation used