amines and amino acids (A2) Flashcards
name this compound
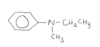
N-ethyl-N-methyl phenyl amine
phenyl is used in the root name and not as a branch because its larger
give the 3 functional groups of amines
primary amines = R-NH2
secondary amines = R1NHR2
tertiary amines = R1NR2R3
therefore primary, secondary and tertiary amines have different functional groups
describe the physical properties of amines
- fishy smell
- 1º and 2º amines that are also short-chained are water soluble due to the polar N𝛿--H𝛿+ bond which can form H bonds with water molecules
- 3º amines are insoluble in water because they do not have the polar N𝛿--H𝛿+ bond
- 1º and 2º amines have BP similar to alcohols
- 3º amines have a lower BP than alcohols because they lack H bonds
describe the ways amines can be synthesised
- nucleophilic substitution of haloalkanes with ammonia
- problem is that you will get a mixture of different amines
- you will not get a 100% yield if you are trying to synthesise a particular amine
- also further distillation required to separate products
- reduction of nitriles to make primary amines
- RCN + 4[H] → RCH2NH2
- reducing agent = LiAlH4 in dry ether (because it reacts vigorously with water)
- reduction of nitrobenzenes makes aromatic amines
- C6H5NO2 + 6[H] → C6H5NH2 + H2O
- because 6 moles of [H] are needed, a very strong reducing agent is required - Sn + HCl
- also NaOH (aq) and heat are needed
describe the chemical properties of amines
- weak bases - only partly dissociate in water
- RNH2 + H2O ⇌ RN+H3 + O-H
- the react with acids to form salts
- RNH2 + HCl ⇌ RN+H3Cl- (ammonium chloride salt)
- they react with carboxylic acids to produce ammonium salts
- RCOOH + RNH2 ⇌ RNH3OOCR
- can acts as nucleophiles due to the LP on N
- nucleophilic substitution to produce 2º, 3º and 4º alkyl salts (which are crystalline ionic solids at room temp)
- excess haloalkane needed for this to happen
- react with acyl chlorides to form amides via nucleophilic addition elimination

in order of strength of basicity:
primary amines > ammonia > aromatic amines
explain why
- primary amines are stronger bases than ammonia because the alkyl group on the primary amine pushes e- density towards the N atom so the LP on N is more available to accept a H+
- the LP on N in aromatic amines is delocalised into the aromatic ring = less e- density around the N atom = LP less available to accept a H+
why are 3º amines weaker bases than 1º and 2º amines?
3º are the weakest bases due to steric hindrance (the 3 R groups prevent the LP on the N atom from accepting H+)
describe the uses of amines
synthesis of drugs
make asodyes and indicators
make condensation polymers
4º alkyl ammonium salts can be used as cationic sufactants in detergents, fabric softener and hair conditioner - they coat the surface of hair/fabric with positive charges and prevent electrostatic forces with negatively charged e-
give the general formula of 2-amino acids
amino group not always on carbon 2 but at a-level we focus more on 2-amino carboxylic acids

describe the physical properties of amino acids
- all amino acids except glycine(2-amino ethanoic acid) show optical isomerism
- natural amino acids are mostly - enantiomers
- medium and short chain amino acids are water soluble as both the NH2 and COOH are polar groups
- higher BP than carboxylic acids/aldehydes/ketones with similar Mr due to twice as many H bonds/dipole-dipole forces between amino acid molecules and ionic bonds between zwitter ions (ionic bonds stronger than intermolecular forces)
describe how amino acids can be synthesised
- most common way is via the hydrolysis of proteins
- protein + (n-1) H2O → amino acids
- using HCl or enzymes as catalysts
- enantiomers will be produced only
- many different ways to synthesise amino acids in a lab depending on the starting product
describe the chemical properties of amino acids
- each functional group keeps its separate chemical properties
- e.g amine group has basic and nucleophilic properties
- NH2 become N+H3 in low pH
- amine group can be acylated to become amide
- carboxyl group has acidic properties
- COOH becomes COO- in high pH
- carboxyl group can be esterified
- therefore amino acids are amphoteric (they can act as both bases and acids)
- also be careful about R groups as they may also participate in reactions
- less acidic than most carboxylic acids and less basic than most amines
- zwitter ion character occurs due to the interaction between the two functional groups
- LP on N accepts the H atom from the carboxyl group within the same molecule
- this forms a zwitter ion which has both a positive charge (on the N atom) and a negative charge (on the O of the carboxyl group)
- amino acids undergo condensation polymerisation to form proteins (secondary polyamides)

what is the isoelectric point
the pH value at which an amino acid precipitates (amino acid molecules become zwitter ions and form ionic bonds between the zwitter ions resulting in a solid)


