Endocrinology Flashcards
What are the three hormones that control the biologically active, free plasma Ca++ concentration in the body? What percentage is in the extra-cellular fluid (ECF)?
- Parathyroid hormone (PTH)
- Calcitonin
- Vitamin D
** 0.1% is in the ECF
What does an increase in free Ca++ do in regards to neuromuscular activity? What about a decrease?
** so what are potential consequences of hypocalcaemia and hypercalcaemia?
* An increase in free Ca++ changes the membrane permeability to Na+– an increase of Ca++ decreases Na+ permeability, resulting in a fewer action potentials and therefore depression of activity
* A decrease of free Ca++ results in over-excitability of nerves and muscles by lowering the threshold with which a response is induced– so a decrease in Ca++ increases Na+ permeability of the cell membrane, resulting in an influx of Na+ moving the resting potential closer to threshold
** hypocalcaemia- can result in muscle spasm, spastic contraction of the respiratory muscles can result in death by asphyxiation
** hypercalcaemia- can cause cardiac arrhythmia and depression of neuromuscular activity
Functions of calcium in the body
- Neuromuscular activity
- Excitation contraction coupling in cardiac and smooth muscle
- Release of products from secretory cells by exocytosis e.g. insulin release
- Tight junction maintenance
- Blood clotting (Ca++ acts as a co-factor in clotting cascades)
What hormones assist in Ca++ absorption in the digestive tract?
PTH and Vitamin D– depends on the Ca++ status of the body.
How is calcium homeostasis maintained?
Goal: to maintain a constant free plasma concentration of Ca++
* Rapid exchange between bone and ECF
* Minor contribution made by urinary excretion of Ca++
What is the goal of PTH? And important actions?
* PTH is the predominant hypercalcemic hormone- increase Ca++ concentration in the palsma– has actions on bone, kidneys, and intestine
- Increase blood calcium conc and decrease blood phosphorous
- increase urinary excretion of phosphorous by diminishing tubular reabsorption in the proximal convoluted tubule
- increase reabsorption of calcium in the distal convoluted tubules, therefore less calcium loss
- Increase the rate of skeletal remodelling and the net rate of boen resorption
- Increase osteolysis and the numbers of osteoclasts on bone surfaces
- increase urinary excretion of hydroxyproline (major component of collagen)
- activate adenyl cyclise in target tissues
- accerlerate the formation of the principle active metabolite 1, 23- OH2D3 by the kidney through a trophic effect on 1alpha-hydroxylase in the mitochondria or renal epithelial cells in the proximal convoluted tubule (1,23-OH2D3= Calcitriol= INCREASES LEVELS OF CALCIUM IN THE BLOOD BY INCREASING UPTAKE BY THE GUT)
What type of cell do parathyroid glands contain? What is secreted?
Chief cells continuously secreting PTH
Where does calcitonin come from? What is it’s role?
Secreted continuously and increases greated in respons to elevated blood calcium– by C cells in the thyroid gland- these cells are distinct from follicular cells of the thyroid that secrete the thyroid hormones.
* Calcitonin acts on bone to decrease entry of calcium into plasma by temporarily inhibiting PTH- stimulated bone resorption
** the actions of calcitonin and PTH are antagonistic on bone resorption, but synergistic on decreasing the renal tubular role resorption of phosphorous
** Calcitonin functions as an emergency hormone to prevent hypercalcaemia during rapid postprandial absorption of calcium AND to protect against excessive loss of calcium and phosphorous from the maternal skeleton during pregnancy
What are the two effects of PTH on Ca++ mobilisation from bone?
* Fast- lost Ca++ from bone fluid– does not involve resorption of bone
* Slow- increased osteoclast resorption (chronic)– increased formation of osteoclasts & transiently decreasing the bone formation activity of osteoblasts
What activates vitamin D? What does the activation of vitamin D result in?
* The kidneys activate vitamin D– this is enhanced by PTH causing a decrease in the reabsorption of PO4 by the kidneys; while enhancing the reabsorption of Ca++
** Vitamin D increases intenstinal absorption of Ca++ (this is an indirect effect of PTH on Ca++ with vitamin D as the intermediary)
Regulation of PTH and Calcitonin Secretion
** primary signal for PTH regulation is plasma free Ca++
** simple negative feedback regulation- no nervous or other hormonal influence
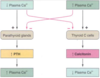
What is this? What happens when it is activated?

Extracellular calcium sensing receptor. This is how changes in plasma Ca++ concentration are detected. It is expressed on parathyroid cells and C cells in the thyroid gland, in the kidney, osteoblasts in bone, hematopoietic cells in bone marrow, placenta, GI mucosa, squamous epithelial cells of the oesophagus. (calcium acting as a hormone has direct effects on the function of many cell types)
- activation of phospholipase C, which leads to generation of the second messengers diacylglycerol and inositol triphosphate
- Inhibition of adenylate cyclase, which suppresses intracellular concentrations of cyclic AMP
What is Vitamin D considered? Why?
* a hormone (aka calcitriol)- as it can be produced in the skin from a precursor related to cholesterol by sunlight and is subsequently released into the blood to act at a distant target site.
** vitamin D must be activated via 2 sequential additions of an -OH group in the liver and then the kidneys
** Vitamin D’s major target is the mucosa of the SI. In the proximal SI it increases active transcellular transport of Ca++ and in the distal part the transport of phosphorous.
Difference in the role of vitamin D and PTH
Vitamin D acts to ensure retention of sufficient mineral ions for mineralisation of bone matrix; whereas PTH maintains the proper ratio of calcium to phosphorous in extracellular fluids.
* a small amount of vitamin D is needed to permit PTH to exert its action on bone
What is PTH hypersecretion?
* occurs in domestic animals in response to a poor Ca++ diet e.g. all meat diet in carnivores
* can also result from a hyper secreting tumour of the parathyroid gland but it is rare
* Consequences can include: reduced excitability of muscle and nervous tissue which leads to muscle weakness and neurological disorders; excessive mobilisation of Ca++ and PO4 from skeletal stores results in thinning of bones, skeletal deformities and increased fracture risk; increase incidence of Ca++ containing kidney stones from excessive Ca++ filtered through kidneys
What are the consequences of a Vitamin D deficiency?
* major cause is impaired intestinal absorption of Ca++, under these conditions PTH maintains plasma Ca++ at the expense of bones. Improper mineralization can lead to soft and deformed bones- rickets in young and osteomalacia in adults

Parturient Paresis (Milk fever, parturient hypocalcaemia)- most common metabolic disorder affecting high producing dairy cattle. Inability of dairy cow to mobilize adequate amounts of calcium results in characteristic symptoms usually obvious 72 hours post-parturition: of restlessness, anxiety, anorexia, uncoordination, and lack of interest in a calf. Incidence increases with age and yield.
** without intervention– cows progress to second stage which is manifest by recumbency– body temp drops– eventually dullness to coma
How does hypocalcaemia in dogs manifest?
Excitement (eclampsia) with restlessness, panting, trembling, muscular tetany and convulsive seizures. Disorder occurs in bitches of small breeds of dogs during lactation.
Why do dogs and cows react differently with hypocalcaemia?
* cows- paresis, dogs- tetany
* differences in functions of neuromuscular junction– the release of acetylcholine and transmission of nerve impulses across the NMJ are blocked by hypocalcaemia in cows (but not in dogs)
* tetany occurs in dogs as a result of spontaneous repetitive firing of motor nerve fibres- owing to the loss of stabilising membrane-bound calcium, nerve membranes become more permeable to ions and require stimulus of a less magnitude to depolarise
What are the two main hormones secreted by the thyroid? Which is the most abundant (>90%)? Which is more potent?
* Thyroxine (T4) (most abundant)
* Tri-iodothyronine (T3)– may be directly synthesized or formed from T4, 3-4xmore potent than T4
What are the effects of thyroid hormones?
- Increase basal metabolic rate in all tissues (except brain, spleen, testes, and lung)– increased mitochondrial size and number, increased protein turnover (both catabolism and synthesis), increased carbohydrate turnover (both catabolism and synthesis), increased lipolysis
- Promote growth in young animals (acting with growth hormone)- thyroid hormone particularly important for normal neurological and musculoskeletal development
- Promote hyperglycaemia through glycolysis, gluconeogenesis and intestinal glucose absorption
- Stimulatory effects on cardiac function- both chronotropic (increases heart rate) and inotropic (increases heart contraction strength)
Regulation of thyroid secretion
* Thyrotropin-releasing hormone (TRH) is released from the anterior pituitary–> TRH stimulates secretion of thyroid- stimulating hormone (TSH) by anterior pituitary–> thyroid secretes T3 and T4
* TSH secretion is suppressed by T4 and somatostatin with minor effects of glucocorticoids and sex hormones


Ectopic thyroid tissue- common incidental finding in ventral neck, mediastinum, and heart base. May become neoplastic.