Endocrine Pancreas Flashcards
Endocrine part of pancreas:
secretes what?
regulates what?
islets of langerhans innervated by what?
secrete insulin, glucagon, somatostatin
regulate glucose, FA, aa, metabolism
islets are innervated by adrenergic, cholinergic, and peptidergic neurons
Beta cells what percent, secrete what
alpha cells what percent, secrete what
delta cells what percent what do they secrete.

How do cells of the islets of langerhans communicate?
Junctions?
blood supply?
-venous blood from beta cells carries insulin to wht cells?

Insulin
main stimulatory of what?
where synthesized?
preproinsulin vs proinsulin?
glucose main stimulatory factor of insulin

what is used as a long term marker of endogenous insulin secretion?
C peptide
Steps of regulation of insulin secretion:
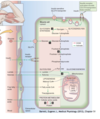
Transport of glucose into the β cell. The β cell membrane contains GLUT2, a specific transporter for glucose that moves glucose from the blood into the cell by facilitated diffusion (Step 1).
2.
Metabolism of glucose inside the β cell. Once inside the cell, glucose is phosphorylated to glucose-6-phosphate by glucokinase (Step 2), and glucose-6-phosphate is subsequently oxidized (Step 3). ATP, one of the products of this oxidation step, appears to be the key factor that regulates insulin secretion.
3.
ATP closes ATP-sensitive K + channels. K + channels in the β cell membrane are regulated (i.e., opened or closed) by changes in ATP levels. When ATP levels inside the β cell increase, the K + channels close (Step 4), which depolarizes the β cell membrane (Step 5). (Refer to Chapter 1 for the complete discussion of why closing the K + channels depolarizes the cell. Briefly, when the K + channels close, K + conductance decreases and the membrane potential moves away from the K + equilibrium potential and is depolarized.)
4.
Depolarization opens voltage-sensitive Ca 2+ channels. Ca 2+ channels, also in the β cell membrane, are regulated by changes in voltage; they are opened by depolarization and closed by hyperpolarization. The depolarization caused by ATP opens these Ca 2+ channels (Step 6). Ca 2+ flows into the β cell down its electrochemical gradient and the intracellular Ca 2+ concentration increases (Step 7).
5.
Increased intracellular Ca 2+ causes insulin secretion. Increases in intracellular Ca 2+concentration cause exocytosis of the insulin-containing secretory granules (Step 8). Insulin is secreted into pancreatic venous blood and then delivered to the systemic circulation. C peptide is secreted in equimolar amounts with insulin and is excreted unchanged in the urine. Therefore the excretion rate of C peptide can be used to assess and monitor endogenous β cell function.

Biphasic secretion of insulin
names
acute, then chronic phases

how does insulin act on its target cells?
Insulin acts on its target cells, as described in the following steps:
1.
Insulin binds to the α subunits of the tetrameric insulin receptor, producing a conformational change in the receptor. The conformational change activates tyrosine kinase in the β subunits, which phosphorylate themselves in the presence of ATP. In other words, the β subunits autophosphorylate.
2.
Activated tyrosine kinase phosphorylates several other proteins or enzymes that are involved in the physiologic actions of insulin including protein kinases, phosphatases, phospholipases, and G proteins. Phosphorylation either activates or inhibits these proteins to produce the various metabolic actions of insulin.
3.
The insulin-receptor complex is internalized (i.e., taken in) by its target cell by endocytosis. The insulin receptor is either degraded by intracellular proteases, stored, or recycled to the cell membrane to be used again. Insulin down-regulates its own receptor by decreasing the rate of synthesis and increasing the rate of degradation of the receptor. Down-regulation of the insulin receptor is in part responsible for the decreased insulin sensitivity of target tissues in obesity and type II diabetes mellitus.

Insulin receptor signaling:
- Insulin binding to receptor triggers what process?
- what does this activate?
- overall end goal

Peripheral uptake of glucose:
- uses what process?
- insertion of what transporter via what pathway cascade?
- types of tissue effective for glucose uptake

Muscle: Insulin actions
- glucose uptake actio due to what?
- glycogen synthesis due to what enzymes?
- glycolysis/carbohydrate oxidation due to what enzymes?
- gluconeogenesis effect
- protein synthesis/breakdown effects
Effect of insulin on muscle. Insulin has four major effects on muscle cells. First, insulin promotes glucose uptake by recruiting GLUT4 transporters to the plasma membrane. Second, insulin promotes glycogen synthesis from glucose by enhancing the transcription of hexokinase (1) and by activating glycogen synthase (2). Third, insulin promotes glycolysis and carbohydrate oxidation by increasing the activity of hexokinase (1), phosphofructokinase(3), and pyruvate dehydrogenase (4). These actions are similar to those in liver; note that there is little or no gluconeogenesis in muscle. Fourth, insulin promotes protein synthesis (5)and inhibits protein breakdown (6).
Alternative intracellular pathways for glucose uptake independent of insulin
-exercise stimulates activation of what?

insulin affect on triglyceride and fatty acid metabolism in adipose tissue
insulin promotes uptake of triglycerides via the activity of lipoprotein lipase, insulin inhibits the hormone sensitive lipase to decrease fatty acid release, insulin promotes conversion of glucose to a-glycerol-p, which promotes conversion of FA to to triglyceride, Insulin promotes uptake of FA to be converted to triglyceride

Summary of insulin actions in
fat
striated muscle
liver

how do these actions influence blood levels
















