Cardiac Function I, II, and III Flashcards
(26 cards)
Cardiac Cycle
- P wave & QRS complex
- Ventricular filling
- Isovolumic contraction
- Ventricular ejection
- Isovolumic relaxation
- P wave & QRS complex
- P wave: atrial depolarization
- QRS complex: ventricular depolarization
- Ventricular filling (diastole)
- Ventricular pressure < pulm/aortic pressure
- Ventricular pressure < atrial pressure
- AV valves open, pulm/aortic valves closed
- Isovolumic contraction (systole)
- Ventricular pressure < pulm/aortic pressure
- Ventricular pressure > atrial pressure
- AV & pulm/aortic valves closed
- Ventricular ejection (systole)
- Ventricular pressure > pulm/aortic pressure
- Ventricular pressure > atrial pressure
- AV valves closed, pulm/aortic valves open
- Isovolumic relaxation (diastole)
- Ventricular pressure < pulm/aortic pressure
- Ventricular pressure > atrial pressure
- AV & pulm/aortic valves closed
Cardiac Cycle
- Passive filling
- Active filling
- End-diastolic volume (EDV)
- End-systolic volume (ESV)
- Stroke volume (SV)
- Ejection fraction (EF)
- Passive filling
- Blood flowing from atria (higher pressure) to ventricles (lower pressure)
- Active filling
- Due to atrial contraction
- End-diastolic volume (EDV)
- Max ventricular volume at the end of filling
- End-systolic volume (ESV)
- Min ventricular volume at the end of ejection
- Stroke volume (SV)
- Volume ejected by teh ventricle during one cardiac cycle
- SV = EDV - ESV
- Ejection fraction (EF)
- SV as a fraction of EDV
- EF (%) = 100 * (SV / EDV)
Cardiac Muscle Structure
- Intercalated disks
- Syncytium
- Sarcomere
- Actin
- Troponin & Tropomyosin
- Myosin
- Titin
- Molecular motor
- Intercalated disks
- Muscle cells are interconnected by intercalated disks
- Allow ions & electrical current to pass through
- Syncytium
- When one cardiac muscl cell gets excited, the AP spreads rapidly to all cell sint eh latticework
- Sarcomere
- Basic unit that gives a striated appearance
- Orderly arrangements of thick & thin filaments
- Actin
- Comprise thin filaments
- Globular (G) actin monomers are linked together in a chain-like structure & form 2 helically arranged polymer strands
- Troponin & Tropomyosin
- Regulatory proteins that regulate cardiac muscle contraction
- Myosin
- Comprise thick filaments
- Dimers consisting of 2 intertwined subunits
- Each subunit has a long tail & a protruding head called a cross-bridge
- Titin
- Large, elastic protein that extends along each thick filament from the M line to each Z line
- Determine the passive mechanical properties of cardiac muscle
- Molecular motor
- Interaciton between actin & myosin is responsible for muscle force generation & muscle shortening
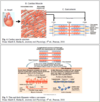
Thin filament activation
- Resting condition
- Electrical stimulation
- Resting condition
- [Ca2+]i is low
- Myosin is blocked from interacting w/ actin
- Electrical stimulation
- Muscle cell depolarization
- Influx of Ca2+ through voltage-gated Ca2+ channels in the sarcolemmal membrane triggers a large release of Ca2+ from the SR –> [Ca2+]i rises
- Ca2+ binds to troponin C
- Comformational change in troponin-tropomyosin complex –> it no longer blocks active sites on actin
- Actin-myosin interaction can take place
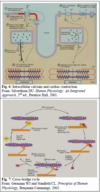
Actin-myosin interaction
- Cross-bridge cycle
- Rigor
- Relaxation
- Key points
- Unbinding of myosin and actin
- ATP enters the ATPase site on myosin
- Causes myosin that’s bound to actin to detach
- Cocking of the myosin head
- ATP hydrolyzed into ADP and Pi
- Energy is captured by myosin (high-energy state)
- Binding of myosin to actin
- Myosin head binds to neighboring actin
- Release of Pi from myosin ATPase site
- Power stroke
- Release of Pi transitions mysoin to the low-energy state
- Myosin head pivots toward the middle of the sarcomere, pulling the thin filament along with it
- Results in force generation and sarcomere (muscle) shortening
- ADP is released from the myosin ATPase site
- Rigor
- Myosin is in the low-energy state, tightly bound to acin
- Myosin is unable to separate until a new ATP molecule binds to the mysoin ATPase site
- Rigor mortis: in the absence of ATP, the cross-bridge cycle gets stuck here
- Relaxation
- Ca2+ unbinds from troponin & is transported back to the SR via the ATP-dependent pump (Ca2+-ATPase)
- Ca2+ is also removed from teh cell in exchange for extracellular Na+ via the Na+-Ca2+ exchanger on the sarcolemmal membrane
- Key points
- Contractile activity originates from the pulling of thin filaments by myosin heads bound to actin (the power stroke)
- The energy needed for this is derived from ATP hydrolysis by myosin ATPase
4 ways to augment the intensity of contraction
- Increasing sarcomere (cell) length
- Alters overlap b/n thin & thick filaments
- Increases actin-myosin interaction
- Affects myofilament Ca2+ sensitivity
- Increasing cytosolic Ca2+ levels
- Cellular Ca2+ handling: higher Ca2+ levels produce greater thin filament activation, greater number of cross-bridges, & more intense contraction
- Increasing thin filament activation
- Changes Ca2+ binding & unbinding to troponin
- Associated w/ cardiac cell remodeling
- Altering kinetic rate constants of cross-bridge cycling
- Number of cross-bridges in the post-power stroke increase
- Associated w/ cardiac cell remodeling
Force-length relationship
- As the initial muscle length is increased via stretching, both initial (or passive) force & peak force increase
- Initial (passive) force: muscle is in the resting or passive condition (not stimulated)
-
Peak developed (active) force: amount of max force generated by the act of active contraction
- Difference b/n peak force & passive force
- Increases w/ muscle length
- Ex. if you stretch a rubber band, you’ll generate higher forces
- Muscle contracts more vigorously as it’s stretched during diastole

Peak developed (active) force-length relationship
- Structural basis: degree of overlap b/n thin & thick filaments
- Length-dependent Ca2+ sensitivity
- Peak developed (active) force increases as muscle length increases
- Structural basis: degree of overlap b/n thin & thick filaments
- Muscle length changes cause sarcomere length to change
- Alters thin-thick filament overlap & size of the pool for myosin-(cross-bridge)-actin interaction
- Sarcomere length = 2.2-2.3: when the actin filaments fully overlap cross-bridges on each side of the myosin filaments
- Sarcomere length > 2.2-2.3: linear decline in force until it becomes 0 at length 3.6
- Sarcomere length < 2.2-2.3: steeper decline in force due to steric interference from double overlap of thin filaments
- Length-dependent Ca2+ sensitivity
- Longer sarcomere lengths –> greater Ca2+ sensitivity
- At a given [Ca2+]i, decreased sarcomere length –> decreased active force

Passive force-length relationship
- Relationship
- Interstitial collagen
- Intracellular titin
- Amount of force needed to stretch the muscle by a certain amount increases as the muscle length is increased
- Interstitial collagen
- Collagen b/n muscle cells
- Increase in collagen –> increased muscle stiffness (steeper slope)
- Ex. myocardial infarction, excessive collagen deposition w/ hypertensive hypertrophy
- Affects muscle stiffness at longer muscle lengths
- Intracellular titin
- Giant elastic protein that runs along thick filaments from Z to M lines
- Affects muscle stiffness at shorter muscle lengths
- Skeletal muscle isoforms are larger & more extensible than cardiac muscle isoforms
- Relaxed cardiac muscle at shorter lengths displays greater passive stiffness than skeletal muscle
- 2 isoforms expressed in cardiac muscle: N2B (less extensible) & N2BA (more extensible)
- Ratio of isoforms correlates w/ cardiac muscle stiffness
- Large forces are required to stretch cardiac muscle to optimal sarcomere length
- Cardiac muscle normally functions on the ascending limb of the force-length relationship

Force-velocity relationship
- Under physiological conditions, muscle shortens as it contracts
- An increase in shortening load decreases the degree, duration, & velocity of shortening
- Max shortening velociyt occurs at the beginning of shortening
- Experiment
- Stimulate muscle attached to a weight
- Muscle increases force until the point where it lifts the weight
- Amount of shortening decreases as you increase the weight
- Afterload: amount of weight muscle is working against
- Inverse relationship b/n initial velocity & shortening load
- Higher load = lower velocity
- Max load: isometric contraction
- Muscle shortening & velocity = 0
- Muscle force is greatest
- Min load: shortening load = 0
- Muscle shortening & velocity is greatest
- Muscle force = 0

Preload, Afterload, & Contractility
- Preload
- Initial/resting muscle (sarcomere) length
- Increased preload –> increased intensity of muscle contraction
- Afterload
- Force against which the muscle has to contract & shorten
- Increased afterload –> decreased velocity & shortening
- Contractility
- Increased contractility –> increased intensity of muscle contraction
- More peak active force is generated at any given length
- Shortening velocity is higher at any given force
- Affected by [Ca2+]i, thin filament activation, & kinetic rate constants of cross-bridge cycling
- Increased contractility –> increased intensity of muscle contraction
Physiological regulation of cardiac muscle contractility
- Sympathetic nervous system
- Sympathetic transmitter NE causes…
- Net effects of NE on muscle contractility
- Parasympathetic (vagal) innervation
- Sympathetic nervous system
- Primary physiological regulator of muscle contractility
- NE increases [Ca2+]i –> increases HR –> increases contracitlity (staircase effect)
- Sympathetic transmitter NE causes…
- Increased influx of Ca2+ through voltage-dependent Ca2+ channels
- Increased Ca2+ release from teh SR
- Increased rate of Ca2+ uptake by the SR
- Increased rate of cross-bridge cycling
- Net effect: increased magnitude of muscle force + augmetned kinetic aspects –> greater muscle shortening
- Net effects of NE on muscle contractility
- Increase force
- Increase shortening
- Increase rate of force development & relaxation
- Parasympathetic (vagal) innervation
- Direct effect on cardiac muscle contractility
- Indirect effect on muscle contractility through vagal influence on HR

Ventricular pressure-volume-flow and muscle force-length-velocity
- Connection b/n ventricles & muscles
- Laplace Law
- Connection b/n ventricles & muscles
- Ventricular volume ~ muscular length
- Ventricular flow ~ muscular velocity
- Ventricular pressure ~ muscular force
- Laplace Law
- σ = (P / 2h) * r = (P / 2h) * (3 / 4π)1/3 * V1/3
- σ = muscle stress
- P = ventricular pressure
- h = ventricular wall thickness
- r = ventricular chamber radius
- V = ventricular volume
- Stress: directly proportional to pressure & volume and inversely proportional to wall thickness
- Dilated ventricle: increased muscle stress
- Hypertensive hypertrophy: opposing effects of increased pressure and hypertrophy (increased wall thickness) may cancel each other
- σ = (P / 2h) * r = (P / 2h) * (3 / 4π)1/3 * V1/3
End diastolic pressure volume relationship (EDPVR)
- Compliance curve of the relaxed diastolic left ventricle
- Slope = contractility
- Increased contractility: slope shifts up & to the left
- Decreased contractility: slope shifts down & to the right

Ventricular Preload
- Best measure
- Factors that determine it
- Venous compliance
- Blood volume
- Resistance to venous return (RVR)
- Intrathoracic pressure (ITP)
- HR via its effects on filling time
- Ventricular passive stiffness
- Posture
- Activity of skeletal muscle
- Best measure
- Ventricular end-diastolic volume (EDV)
- Determines muscle & sarcomere length
- Factors that determine it
- Venous compliance
- Increase compliance –> decrease preload
- Blood volume
- Increase blood volume –> increase preload
- Resistance to venous return (RVR)
- Increase RVR –> decrease preload
- Intrathoracic pressure (ITP)
- Increase ITP –> decrease preload
- HR via its effects on filling time
- Increase heart rate –> decrease filling time –> decrease preload
- Ventricular passive stiffness
- Increase stiffness –> decrease preload
- Posture
- Supine to upright –> decrease preload
- Activity of skeletal muscles
- Increase activity –> increase preload
- Venous compliance
Ventricular Afterload
- Best measures
- Effect of ventricular contractile activity
- Mechanical opposition to ejection (afterload) is determined by…
- Best measures
- Ventricular pressure during ejection
- End-systolic ventricular pressure (ESP) ~ mean arterial pressure (MAP)
- Total peripheral resistance (TPR)
- Effect of ventricular contractile activity
- Increase ventricular contractility –> increase cardiac output –> increase MAP –> increase afterload
- Mechanical oppositoin to ejection (afterload) is determined by…
- Extrinsic factors (ex. TPR)
- Intrinsic factors (ex. ventricular contractility)
Ventricular Contractility
- Best measure
- Following epinephrine administration
- Alternative indices of ventricular contractility
- Best measure
- End systolic pressure volume relationship (ESPVR)
- Following epinephrine administration
- ESPVR slope is increased w/o changing intercept –> increased contractility
- Ventricle produces more pressure for a given volume & generates more stroke volume for a given afterload (end-systolic pressure)
- Alternative indices of ventricular contractility
- Ventricular stroke volume - EDV/EDP relationship (Frank-Starling)
- Venticualr stroke work - EDV/EDP relationsihp (ventricular function curve)
- Ventricular max rate of pressure development - EDV relationship
- Increased contractility shifts relationship leftward so ventricle can generate more stroke work for a given preload

How stroke volume depends on ventricular preload, afterload, and contractility
- SV = EDV - ESV
- Increase preload –> increase EDV –> increase SV
- Increase afterload –> increaes ESV –> decrease SV
- Increase contractility –> increase SV

Physiological regulation of ventricular contractility
- Primary regulator: sympathetic nervous system via NE & Epi release
- Increase SNS –> increase HR –> increase [Ca2+]i –> increase ventricular contractility (staircase effect)
- Increase NE –> increase rate of force development –> increase shortening velocity during ejection –> increase rate of relaxation –> increase contractility
- SNS allows heart to maintain a high cardiac output during exercise
- Increase HR –> decrase filling period –> adversely affect EDV & stroke volume
- However, increase HR –> increase shortening velocity to maintain or increaes stroke volume
- Parasympathetic nervous system
- Little direct effect on ventricular contractility
- Release Ach –> increase PNS activity –> decrease HR –> indirectly decrease ventricular contractility
Physiological relevance of the Frank-Starling relationship
- Frank-starling relationship
- If right heart output is transiently greater than left heart output
- In a diseased heart (ex. dilated cardiomyopathy)
- Frank-starling relationship
- Increase preload –> increase contraction intensity
- Cardiac muscle operates on ascending limb b/c shorter & less extensible cardiac titin resists myocyte overextension
- Slope is steep so small changes in preload –> large changes in contraction intensity
- Stroke volume is less sensitive to changes in afterload
- Intrinsic mechanism for maintaining equal right & left ventricular outputs
- If right heart output is transiently greater than left heart output
- Blood pools in pulmonary circulation
- Increase pulmonary venous pressure
- Increase left atrial pressure
- Increase left ventricular filling & EDV (preload)
- Increase left heart output
- In a diseased heart (ex. dilated cardiomyopathy)
- Reduced steepness of the Frank-Starling relationship
- Stroke volume becomes more sensitive to changes in afterload

Similarities between ventricular and muscular mechanical behaviors
- Increase in volume (muscule length) –> more intense contraction (higher pressures)
- ESPVR ~ ventricular peak (active) pressure-volume relationship
- EDPVR ~ ventricular passive pressure-volume relationship
- Similar effects of changes in preload, muscle length shortening, & ejection volume (stroke volume)
- Similar effects of increased contractility & afterload

Ventricular passive mechanical behavior
- Characterized by…
- Myocardial composition
- Ratio of EDV and ventricular muscle mass
- Characterized by the end diastolic pressure-volume relationship
- Slope: nonlinear, increases as EDV increases
- Myocardial composition
- Increased collagen –> leftward shift –> increased stiffness
- Shift of titin from N2BA (more extensible) to N2B (less extensible) –> leftward shift –> increased stiffness
- Ratio of EDV and ventricular muscle mass
- Increased muscle mass in idiopathic hypertrophic subaortic stenosis –> increased EDP –> decreased filling –> decreased EDV
- Coronary artery disease –> increased collagen –> increased stiffness

Stroke Work and Myocardial Oxygen Consumption (MVO2)
- Stroke work
- Ventricle does external work during a cardiac cycle when it generates pressure to eject the stroke volume
- SW = area enclosed by the P-V loop confined within ESPVR & EDPVR curves
- A = end-diastole
- B = onset of ejection
- C = end-systole
- D = onset of filling
- Width = SV = EDV - ESV
- Height = ESP - EDP = MAP - EDP
- SW = (MAP - EDP) * SV
- Myocardial oxygen consumption (MVO2)
- MVO2 = input energy used to generate ATP for contractions
- The same SW can result in different MVO2s
- Higher MVO2: high MAP, low SV
- Lower MVO2: low MAP, high SV

Pressure-Volume Area (PVA)
-
Pressure-volume area (PVA): total mechanical energy generated by the ventricle
- Stroke work (SW): external energy
- End-systolic potential energy (PE): internal energy, dissipated as heat
- Increase PVA –> increase MVO2





