Block 1 Flashcards
(108 cards)





Sperm maturation arrest; do not see any 2’ spermatocytes or spermatids towards the center of the seminiferous tubule.



Cut surface of normal testes

Testicular torsion: twisting of the spermatic cord, obstruction of thin-walled veins leads to hemorrhagic infarction; usually due to congenital failure of testes to attach to inner lining of scrotum (within processus vaginalis)

Hemorrhagic necrosis seen in testicular torsion

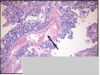
Cryptorchidism: undescended testes; fail to descend into scrotal sac;
complications: testicular atrophy, infertility, and increased risk for seminoma (CA).

Cryptorchidism: see testicular atrophy (yellow arrow)
Bottom L: normal seminiferous tubule full of developing spermatogonia
Bottom R: cryptorchid seminiferous tubule; see no spermatogonia/spermatids –> infertility

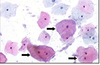
Seminoma:
Most common type of GCT
Large uniform “clear cell” tumor cells (red arrows)
Lymphocytic infiltration (green arrow)
Fibrous septa (yellow arrow)
Do NOT have hemorrhage or necrosis

Spermatocytic seminoma: rare, seen in older pts (54+), doesn’t arise from intratubular germ cell neoplasia
3 cell types:
1) small lymphocyte-like cells: yellow arrow
2) intermediate cells: red arrow
3) giant cells w/ 1+ nuclei: green arrow
Excellent prognosis; not related to cryptorchidism, serum tumor markers not elevated, usuall bilateral

Embryonal carcinoma:
gross path: hemorrhagic, necrotic, poorly circumscribed
histo: large highly pleomorphic cells; lots of pink cytoplasm; overlapping/indistince cell membranes
Poorest prognosis of all GCT’s; see elevated beta-HCG or AFP
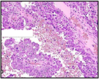
Embryonal carcinoma w/ papillary growth; large pleomorphic cells, indistince cell membrane, lots of overlap; hemorrhagic
poorest prognosis

Yolk sac tumor; most common testicular tumor in kids/infants; see microcystic pattern on histo with multiple intercellular holes (“sieve-like” pattern)
tumors secrete AFP, so see elevated serum levels

Yolk sac tumor: relatively uniform cells with clearish pink/vacuolated cytoplasm;
see Shiller-Duval bodies: (yellow arrow) central BV surrounded by tumor cells; looks like primitive glomeruli
Hyaline-like globules: (black arrows) contains AFP and alpha1-antitrypsin

Mature teratoma: see cartilage (red “A”), ducts/glands (yellow arrow), and hair follicles (black arrows)
Made of 1+ tissues from different germinal layers
2 age peaks: <4 y.o and 20’s-40’s
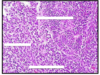
Immature teratoma: undifferentiated spindle cells, primitive small round blue cells; poorly differentiate, poorer prognosis.
pre-pubertal teratoma in males is BENIGN, post-pubertal teratomas in males are MALIGNANT

Choriocarcinoma: tumor of syncitiotrophoblasts and cytotrophoblasts; grossly appears as hemorrhagic tumor; on histo see areas of hemorrhage
rarely pure tumor, usually seen in mixed GCT.
Marked elevation in beta hCG

Choriocarcinoma:
A) syncitiotrophoblasts: large multinucleated cells with pink cytoplasm
B) cytotrophoblasts: polygonal cells with clear cytoplasm, bland nucleus, well define border
C) beta-HCG + stain of choriocarcinoma

Mixed GCT: most common after seminoma; prognosis based on worst component (i.e. embryonal)

Left: hypospadias—urethral opening on ventral surface of penis; 1/300 live births
Right: Epispadias: abnormal urethral opening on dorsal aspect of shaft; even rarer

Peyronie’s dz: localized fibromatosis of penile shaft resulting in painful erections

Penile Infections:
Left: HSV
Right: Syphillis chancre

Condyloma accuminata (genital warts); due to HPV 6 & 11