Biochemistry Flashcards
What is glycogenesis?
Synthesis of glycogen from freely available glucose
What is glycogenolysis?
Breakdown of glycogen to form glucose
In which 2 organs is glycogen present?
Liver and muscle cells
What is the difference between liver and muscle glycogen?
Liver glycogen is broken down between meals and released into the blood stream to maintain blood glucose levels; while muscle glycogen is broken down into glucose but can’t be released into the bloodstream (only used by muscle cells during bursts of physical activity)
When does glycogenolysis occur?
Between meal times
What is gluconeogensis?
Generation of glucose from certain non-carbohydrate carbon substrates
When does gluconeogenesis normally occur?
Overnight when hepatic glycogen is depleted
What are the 3 main sources of blood glucose?
1) Dietary carbohydrates 2) Glycogenolysis 3) Gluconeogenesis
Which kind of bond joins glucose monomers in glycogen?
α 1-4 glycosidic links
Which kind of bond joins branches in glycogen?
α 1-6 glycosidic links
What is glycogenin?
Protein lying at the centre of the branches structure of glycogen. Has catalytic properties, and can add glucose monomers to itself
What is one of the limitations of glycogen synthase (enzyme responsible for synthesising glycogen)?
It can’t start making glycogen on its on, it can only add glucose residues to existing glycogen chains. A glycogen ‘primer’ containing at least 4 glucose residues is required - often primer is added to glycogenin.
What is the starting substrate of glycogen synthesis?
Glucose-6-phosphate
Which enzyme is responsible for converting glucose to glucose-6-phosphate at the beginning of both glycolysis and glycogenesis?
Hexokinase
What are the overall steps in glycogenesis?
1) Glucose-6-phosphate convened to glucose-1-phosphate by phosphoglucomutase
2) Glucose-1-phosphate converted to UDP-glucose by UDP-glucose pyrophosphorylase
3) Glycogen synthase then takes the glucose part of UDP-glucose and covalently bonds it onto existing glucose chains

What is the role of glycogen synthase?
Synthesises glycogen from UDP-glucose by adding one glucose molecule from UDP-glucose to glycogen at a time. (can only extend a chain of glycogen, can’t start new molecules)
Which enzyme is responsible for introducing branches to glycogen?
Transglycosylase
Which enzyme catalyses glycogenolysis?
Glycogen phosphorylase
What is the reaction equation for glycogenolysis?
[glucose]n + phosphate (Pi) -> glucose-1-phosphate + [glucose]n-1
What are the steps for glycogenolysis?
1) Removal of a glucose-1-phosphate from glycogen by glycogen phosphorylase
2) Conversion of glucose-1-phosphate to glucose-6-phosphate by phosphoglucomutase (same as forward reaction)
3) In liver: Glucose 6-phosphate can be de-phosphorylated using glucose-6-phosphatase to form free glucose, and the resulting glucose released into the blood stream
In muscle: Glucose 6-phosphate CANNOT be de-phosphorylated but instead is used to provide energy via glycolysis and the TCA cycle, so can only feed that muscle cell

What is the overall relationship of glycogenesis and glycogenolysis?
They are they opposites of each there, with slightly different enzymes in some steps

Which enzymes regulate both glycogenesis and glycogenolysis?
Insulin, glucagon, adrenaline and cortisol
Which hormones stimulate glycogenesis by stimulating glycogen glycogen synthase?
Insulin (hormone of fed state)
Which hormone stimulates glycogenolysis by stimulating glycogen phosphorylase?
Glucagon (hormone of starving state), adrenaline and cortisol
What are glycogen storage diseases characterised by?
Group of diseases with increased glycogen deposits in liver or muscle or both
What are the precursors of gluconeogenesis?
- lactate - synthesised by skeletal muscle from glycolysis under anaerobic conditions
- amino acids - derived from muscle protein by proteolysis
- glycerol - derived from triglycerides by lipolysis in adipose tissue
Where does the energy for gluconeogenesis come from?
Initially from oxidation of fatty acids released from adipose tissue, but can also be released from the breakdown of body protein
Where does gluconeogenesis mostly occur?
Mainly in the liver, with small amounts in the kidneys
What is gluconeogenesis essentially the reverse of?
Glycolysis: three essentially irreversible reactions, catalysed by the following enzymes: • hexokinase • phosphofructokinase • pyruvate kinase
Why is gluconeogenesis not the exact opposite of glycolysis?
The 3 reactions in glycolysis are irreversible, so several special reactions are required to bypass them, using 4 unique liver enzymes
How many ATP are required for gluconeogenesis?
6 ATP
What is the Cori Cycle?
Synthesis of glucose from lactate formed in fast-twitch muscle under conditions of heavy exercise, particularly anaerobic.
What are the steps in the Cori Cycle?
1) Muscles produce lactate from pyruvate 2) Blood transports lactate to liver 3) Liver converts lactate back to glucose 4) Glucose released into bloodstream
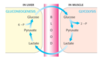
What are the advantages and disadvantages to the Cori Cycle?
Advantages: Means the body isn’t exposed to acidity of lactate for longer than needed, and switches the metabolic burden from muscle to other organs Disadvantages: Energy expensive - uses more ATP than it makes
Which two classes can amino acids be grouped into in terms of metabolism?
- Ketogenic – cant be used for making new glucose. Are degraded into actual-CoA and can be converted to pyruvate then to oxaloacetate forming ketone bodies or fatty acids
- Glucogenic – can be used as precursors in gluconeogenesis. They are degraded into pyruvate of TCA intermediates so can be made into glucose as acetyl groups, but only if oxaloacetate is present.
At which two levels can glycolysis and gluconeogensis be regulated?
1) System level - hormones e.g. insulin and glucagon 2) Individual cell level - via allosteric effectors e.g. AMP, ATP, citrate, fructose-2,6-biphosphate
What does increased fat intake without appropriate energy expenditure lead to?
• increase in numbers of adipocytes • more fat deposits within the adipocytes
What is fat required for?
- As an energy source - For essential fatty acids (once that can’t be made by the body) - For fat-soluble vitamins (e.g. Vit A, D, E and K)
What are the 3 main categories of lipids?
Simple lipids (e.g. fatty aids, triglycerides), compound lipids (e.g. phospho-, glyco-lipids, lipoprotein) and steroids (e.g. cholesterol and steroid hormones)
What are the 3 main types of fatty acids?
• saturated (no double bonds) • unsaturated (one double bond) • polyunsaturated (several double bonds)
What are the main products of fat digestion?
- glycerol - fatty acids - monoglycerides
What happens to the fatty acids and monoglyceride products of fat digestion?
Short and medium length fatty acids enter the portal blood. While longer chain fatty acids and monoglycerides are re-synthesided into trip-glycerides and become chylomicrons (lipoprotein) before entering the lymph and then blood stream
What is fatty acid oxidation (lipid metabolism)?
What happens to a fatty acid once it has been digested in order for it to be used for energy generation
What must happen to fatty acids before then can be oxidised, and where does this occur?
Activation: must be converted to CoA derivatives (acyl-CoA) by combining with acetyl-CoA; this occurs in the cytoplasm
Following activation in the cytoplasm, where does the remainder of fat oxidation occur, and how does the acyl-CoA formed in activation get there?
It occurs in the mitochondrial matrix, and the acyl-CoA gets there via the cartinine shuttle
What is beta-oxidation and what is its yield?
Cycle of reactions in an oxidative process using acyl-CoA from fatty acids as its substrate. Cycle repeats 8 times so produces: - 9 acetyl-CoA, 8 FADH2, 8 NADH + 8 H+
What is the purpose of beta-oxidation?
To provide acetyl-CoA which can be oxidised in the TCA cycle to CO2, and also provide electron carriers (FADH2, NADH) to be oxidised in oxidative phosphorylation for ATP generation. (Basically fatty acids act as alternatives to glucose as energy substrates in TCA cycle)
How is the glycerol produced in fat digestion broken down?
- 1) Activated to glycerol-3-phosphate by glycerol kinase - 2) Dehydrogenated to dihydroxyacetone phosphate, which is a normal intermediate of carbohydrate metabolism
How are ketone bodies associated to fat digestion?
They are formed in the liver mitochondria during beta-oxidation from acetyl-CoA.
What is the important of ketone bodies in energy metabolism?
Important molecules of energy metabolism for heart muscle and renal cortex as they are converted back to acetyl-CoA, which enters TCA cycle where they are oxidised. Therefore an alternative way for the body to move energy around the system
How are ketone bodies involved in starvation or diabetes?
- Oxaloacetate is consumed for gluconeogenesis
- Fatty acids are oxidised to provide energy which generates large amounts of acetyl-cos
- Acetyl-CoA is therefore converted to ketone bodies to get rid of it (as can’t enter TCA as oxaloacetate isn’t available as intermediate)
- High levels in blood
- Becomes too much for extrahepatic tissue (i.e. heart, brain, etc.), leading to acidosis
When does lipogenesis occur?
Occurs when the body obtains more energy than is actually needed at the time, so is converted into stored fat
Where does de novo synthesis of fatty acids occur?
In liver (mainly), but also kidney, mammary glands, adipose tissue and brain
What happens when excess carbohydrate is taken in?
1) conversion to fatty acids and triglycerides in the liver 2) free fatty acids are transported in plasma bound to albumin 3) triglycerides formed in the liver are transported to adipose tissue by VLDL for storage
What are the steps of lipogenesis?
1) Dietary starch is broken down into glucose 2) Glucose is converted to pyruvate 3) Pyruvate is converted to acetyl-CoA by pyruvate dehydrogenase complex in the mitochondrial matrix 4) Citrate groups then transport acetyl-CoA into the cytoplasm 5) Acetyl-CoA then acts as the substrate for fatty acid synthesis 6) Fatty acids are then esterified into triglycerides

Where does lipogenesis (fatty acid synthesis) from acetyl-CoA occur?
In the cytoplasms of liver cells
Where does beta-oxidation occur?
Mitochondrial matrix (common exam question)
What are the overall steps of fat oxidation (lipid metabolism)?
1) Activation: Fatty acids must be converted to CoA derivatives (acyl-CoA) in the cytoplasm 2) Acyl-CoA is then transported into the mitchochondrial matrix by cartinine shuttle 3) Beta-oxidation then occurs as a cycle of reactions using acyl-CoA as its substrate to produce: 9 acetyl-CoA, 8 FADH2, 8 NADH + 8 H+ 4) Acetyl-CoA then enters the TCA cycle while the electron carriers generate ATP via oxidative phosphorylation
What is the intermediate step between acetyl-CoA being converted into fatty acids in lipogenesis?
Activation of acetyl-CoA to malonyl-CoA by acetyl-CoA carboxylase
Which enzyme then takes malonyl-CoA and converts it to fatty acids in lipogenesis?
Fatty acid synthase
What must be present for triglycerides to be synthesised?
Glycerol-3-phosphate
True or False: Some amino acids can also be converted to acetyl-CoA to be broken down in the TCA, as well as fatty acids and pyruvate
True
Which amino acids are degraded in the TCA cycle and why?
Those which aren’t used as protein building blocks, as there isn’t a storage form of amino acids
What is the main site of amino acid degradation?
Liver
Where do the amino acids being degraded come from?
Absorption from the diet or as part of protein turnover
What is the main issue of amino acid breakdown?
They all contain nitrogen, so ammonia are produced which can be toxic at high concentrations. So these need to be safely excreted.
Which molecules are used to safely excrete nitrogen products?
- Urea (80% of nitrogen) - Uric acids - Creatinine - Ammonium ion
What are the steps in urea formation?
1) Transamination: Amino group of an amino acid is transferred to a keto acid, which is normally ketoglutarate, forming glutamic acid (occurs in any tissue) 2) De-amination: Removal of amino group from glutamic acid (can only occur in the liver) 3) Free ammonia ion is formed which enters the urea cycle, forming urea

What happens to the remaining carbon skeletons of the amino acids once the amino (nitrogen) group has been removed?
They are coverted into major metabolic intermediates, either glucose or are oxidised in the TCA cycle (depending on if they are ketogenic or glycogenic)
What are 3 inherited disorders related to abnormal amino acid degradation?
- Acaptonuria - Maple syrup urine disease - Phenylketonuria (All relate to abnormal breakdown of specific amino acids)
What occurs in urea cycle disorders?
Defect in urea cycle enzyme, resulting in the accumulation of urea cycle intermediates
What is the treatment for urea cycle disorders?
- Low protein diet - Drugs which remove nitrogen - Gene therapy in hepatocytes
What are the main proteins found in the plasma?
Albumin (main) and α, β and γ globulins (immunoglobulins)
What are the main overall functions of plasma proteins?
- Maintenance of oncotic or colloid pressure - Transport of hydrophobic substances - pH buffering - Enzymatic e.g. blood clotting -Immunity
What is the main role of α globulins?
-Transport lipoproteins, lipids, hormones and bilirubin and act as retinol binding protein (transports vitamin A)
What is the main role of β globulins?
Involved in transferrin (transports Fe3+) and fibrinogen (inactive form of fibrin)
What at the main roles of albumin?
Main determinant of plasma oncotic pressure, and provide multiple binding sites for hydrophobic molecules e.g. fatty acids, bilirubin, thyroid hormones and exogenous substances e.g. aspirin
How is iron transported and stored?
Transported as ferric ion (Fe3+) bound to transferrin, and stored in cells bound to ferritin
How is copper transported?
Bound to ceruloplasmin
How are hydrophobic hormones such as steroid hormones and T3/T4 thyroid hormones transported?
- Transported in the circulation bound to specific transport molecules e.g. thyroxine bound to thyroid-binding globulin and cortisol bound to cortisol-binding globulin
What are the 5 categories of lipoproteins?
Chylomicrons, VLDL, IDL (intermediate), LDL and HDL
What is the function of chylomicrons?
Transport of exogenous fat to liver
What is the function of VLDL?
Transport of endogenous fat to peripheral cells
What is the function of IDL?
LDL precursor
What is the function of LDL?
Cholesterol transport to peripheral tissues
What is the function of HDL?
Reverse cholesterol transport (removes excess cholesterol from cells and transport it back to the liver to be excreted)
What vitamins and metal ions does the liver store?
Vitamins A, D and B12, and also iron (with ferritin)
Which 3 important classes of biologically active compounds is cholesterol a precursor of?
Bile acids, steroid hormones and vitamin D
What is the main site of cholesterol synthesis?
Liver (as well as intestine, adrenal cortex and gonads - nearly any cell can synthesis cholesterol)
What does the initiation step involve and which enzyme catalyses this? Why is this clinically important?
Initiation step involves acetyl-CoA conversion to mevalonic acid, catalysed by HMG-CoA reductase. This is clinically important as statins target HMG-CoA reductase to limit cholesterol production.
How do you determine the yield of beta oxidation??
1) Determine the number of cycles that will occur: Number of acyl groups divided by 2 and minus 1 (e.g. Cn/2 -1-
2) Multpily the yield of 1 cycle, by this number (1 acetyl CoA + 1 FADH2 + 1 NADH + H+ + 1 fatty acyl-CoA)
What would the yield be for the beta oxidation of a C14 fatty acid?
1) Number of cycles: C14/2-1 = 7 cycles
2) 7 x yield of 1 cycle = 7 acetyl-CoA, 6 NADH +H+ , 6 FADH2
True or False: Ketons diffuse into the bloodstream
True
True or False: Ketones are toxic to peripheral tissues which prefer glucose
False, peripheral tissues prefer glucose, but ketone bodies are useful in certain conditions
True or False: The first step of fat catabolism is lipolysis
True
True or Falee: In fat catabolism, fatty acids first have to be activated to acetyl-CoA
False, they must be activated to acyl-CoA
True or False: Fatty acids can be used for gluconeogenesis
False, the starting point of gluconeogenesis is pyruvate. While beta oxidation instread generates acetyl-CoA exclucively
Where does fatty acid synthesis mostly occur?
Liver
What is the rate limiting enzyme in fatty acid synthesis?
Acetyl-CoA carboxylase- Converts acetyl-CoA to malonyl-CoA


