BCM Phys Lecture 1: Water, pH- Acid/Base Flashcards
What kind of bonds form between water molecules
HYDROGEN BONDS
Explain why water molecules are very polar.
Oxygen has a higher electron affinity/stronger attraction for shared e- than H does.
O has a partial negative charge. H has partial positive charge.

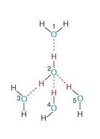
Five molecules of water form to create a ______ structure.
Tetrahedral structure by H bonding.
What are some molecules that can H bond?
O, N, F
What kind of water molecule organization occurs in ice?
Hexogonal water formation, which is a much more open structure. Ice is less dense than water which is why it floats
Define the following terms:
- Electrolyte
- Amphipathic
- Cation
- Anion
Electrolyte: Dissociation of Molecules in water
Amphipathic: compounds that have both polar and nonpolar groups
Cations: positively charged ions
Anions: negatively charged ions
In the partial dissociation of a weak electrolyte, what is the Keq’ eqn?
Keq’ = [H+][A-]/[HA]
Water is a strong/weak electrolyte? How does water dissociate and what is the corresponding eqn?
Water is a WEAK electrolyte.
HOH<-> H+ + OH-
Keq = 1.8 x 10^(-16)
Note: [OH-][H+] = 1.0 x 10^ -14
Proton donor is a _____.
Proton acceptor is a ______.
Proton donor is a weak acid
Proton acceptor is a weak base.
Describe the relationship between [H+], pH, and pOH
Note: pH = -log[H+]
pH+pOH= 14
10-pH = [H+]
What are the pH values/ranges for some of the biological fluids? Example: blood, gastric juice, pancrease, urine

How can you use Keq to identify the stregnth of an acid?
The tendency of the conjugate acid to release a proton can be assessed from Keq’. The smaller the value of Keq, the less tendency to give up the proton (and the weaker the acid)
What is the Henderson Hasselbach equation?
Note: there are equal amounts of conjugate acid and conjugate base if the pH equals the pKa

Give the equation for carbonic acid. What is really treated as the acid? And then what is the important pKa value that we glean from it to solve HH problems.
Carbonic acid does not last long AT ALL, thus CO2 is essentially treated as the acid.
The important pKa in this equation is that:
([H+][HCO3-])/[CO2] has a pK value of 6.1.

Blood buffers try to keep the pH of blood at ____.
Name the blood buffers.
What happens when the bases in blood are used up?
pH around 7.4
HCO3- (bicarb), Hemoglobin, and HPO4- are blood buffers
When the bases are utilized, blood pH will drop significantly
The concentrations of both ____ and ___ and pH of blood are therefore important values in clinical practice to determine acid/base status of patients.
pH less than ____ is acidosis
pH greater than _____ is alkanosis.
Concentrations of HCO3- and CO2
pH < 7.35 is acidosis
pH > 7.45 is alkanosis
Metabolic acidosis occurs when_____.
Metabolic alkanosis occurs when____.
Metabolic acidosis: excess/buildup of organic acids (like ketone bodies in diabetes, hypoxia) OR loss of HCO3- like in diarrhea, uremia, chronic kidney failure
Metabolic alkanosis: retention of HCO3- and ingestion of bases
Respiratory acidosis occurs when______.
Respiratory alkanosis occurs when_____.
Respiratory acidosis: retention of CO2 (like in emphysema, asthma, polio, morbid obesity)
Respiratory alkanosis: hyperventilation and OD on aspirin
What are the two transport systems in the human body that transport CO2 to the lungs?
What’s the percent of total Co2 produced by cells and transported to the lungs for each system respectively?
There is “isohydric transport” of CO2, where CO2 is transported as HCO3- (bicarb). Roughly 70-80 % of CO2 produced by cells is transported to the lungs via this system.
The other system is “transport of CO2 as Carbamino-Hb”, and roughly 15-20% of CO2 produced by cellss is transported to the lungs via this system.


