Alloys Flashcards
1
Q
How are alloys created?
A
By mixing two metals together or a metal and a non-metal.
2
Q
Why does pure iron tend to be bendy?
A
- Straight from the blast furnace is only 96% iron (other 4% is impurities such as carbon).
- Impure iron is used as cast iron. Handy for making ornamental railings. ⇒ Not many other uses because it’s brittle
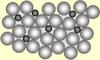
- All impurities are removed from most of the blast furnace iron ⇒ Regular arrangement of identical atoms ⇒ Layers of atoms can slide over eachother making the iron soft and easily shaped. Iron is too bendy for most uses.

3
Q
A
4
Q
Uses of alloys
A
- Bronze is harder than copper - good for making metals and statues
- Aluminium has a low density- alloyed with small amounts of other metals to make it stronger
- Pure gold is too soft. Metals suchas zinc, copper, silver and nickel are used to harden the “gold”
- cupronickel is hard and corrosion resistant. It is used to make “silver” coins.