6: Cardiomyopathy, Myocarditis, Pericarditis, CHD Flashcards
what is: Heterogeneous group of disease associated with mechanical/electrical dysfunction usually exhibiting inappropriate ventricular hypertrophy or dilatation
CARDIOMYOPATHY
3 types of cardiomyopathy;
which is most common?
- dilated** (most common, 90% of cases)
- hypertrophic
- restrictive
dilated cardiomyopathy:
define, epidemiology
- Progressive cardiac dilation and contractile dysfunction, usually with hypertrophy
- Epi:
- Usually diagnosed at end-stage
- **Most common (90% of cases)

what are the acquired causes of dilated cardiomyopathy?
- Myocarditis
- TOXICITIES ( alcohol, chemotherapy - doxorubicin, cobalt ingestion)
- Pregnancy (peripartum cardiomyopathy – late pregnancy or months postpartum)
- Stress-provoked – persistent tachycardia, hyperthyroidism
- Iron overload – hereditary hemochromatosis, multiple transfusions
how many cases of dilated cardiomyopathy are due to genetic causes?
what are the genetic causes?
- Genetic causes (20-50% of cases)
- causes
- >50 genes have been implicated
- Autosomal dominant most common
- Most common mutations affect cytoskeletal proteins (desmin) / proteins linking sarcomere to cytoskeleton (α-cardiac actin)
- X-linked – mutations in dystrophin

Clinical features of Dilated Cardiomyopathy
- Typical pt: dx b/w 20-50 years
-
Dyspnea, easy fatigability
- Over half die within 2 years
- Ineffective contraction –> low ejection fraction (<25% at end stage)
- Secondary mitral regurgitation
- Embolism
- Cardiac arrhythmias
gross anatomical changes w/ DILATED CARDIOMYOPATHY?
- Heart enlarges 2-3x normal
- Flabby (all chambers are dilated)
- Mural thrombi often present - source of thromboemboli

what are the microscopic/ histological changes associated with DILATED CARDIOMYOPATHY?
- myocyte hypertrophy with enlarged nuclei OR
- Attenuated myocytes that are stretched out
- interstitial fibrosis

what are the causes of ARRHYTHMOGENIC RIGHT VENTRICULAR CARDIOMYOPATHY?
- Autosomal dominant – genes encoding desmosomal junctional proteins (plakoglobin) and proteins that interact with desmosomes (desmin)
- Right-sided HF and rhythm disturbances
- Cause of sudden cardiac death
- Right ventricular wall is severely thinned (fatty replacement and fibrosis)
- Left-sided involvement may occur

HYPERTROPHIC CARDIOMYOPATHY:
what is the pathological change, and what aspect of heart function does this affect?
- Change: Thick-walled, heavy, hypercontractile heart
- Effect: Defective compliance/ diastolic filling and ventricular outflow obstruction (1/3rd of cases)
More rare, incidence is 1 in 500

what is the pathogenesis of Hypertrophic Cardiomyopathy?
(hint: autosomal dominant pathology)
-
Missense mutations in genes encoding sarcomeric proteins –> hypertrophic cardiomyopathy
- >400 mutations in 9 genes
-
β-myosin heavy chain > myosin-binding protein C and troponin T
- 70-80% of all cases
what are the gross anatomical changes in hypertrophic cardiomyopathy?
- Massive myocardial hypertrophy w/o ventricular dilation
- Ventricular septum > left ventricular free wall (asymmetric septal hypertrophy)
- Small, banana-like configuration of LV cavity
- LV outflow tract w/ fibrous endocardial plaque
- Anterior mitral leaflet is thickened -> Make contact with each other during ventricular systole
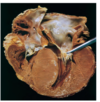
what are the microscopic changes in hypertrophic cardiomyopathy?
- massive myocyte hypertrophy
- haphazardly arranged bundles of myocytes
- interstitial fibrosis

will patients with hypertrophic cardiomyopathy have a family hx of the same condition?
YES; pts will likely have family history of cardiac dysfunction causing death;
this is an autosomal dominant condition caused by missense mutations in genes encoding sarcomeric proteins
which type of cardiomyopathy is associated w/ SUDDEN DEATH IN ATHLETES?
HYPERTROPHIC CARDIOMYOPATHY;
Sudden death - cause of death for 1/3rd of athletes under 35 years
what factors of hypertrophic cardiomyopathy lead to focal myocardial ischmia?
- massive hypertrophy
- high LV chamber pressure
- compromised intramural arteries
clinical features of hypertrophic cardiomyopathy?
- Exertional dyspnea (from compromised cardiac output)
- Harsh systolic ejection murmur
- Focal myocardial ischemia
And sudden cardiac death in athletes
define: sudden cardiac death
(hint: timing/sxs)
Unexpected death from cardiac causes either:
- without symptoms or
- within 1-24 hours of symptom onset
what disease accounts for 80-90% of cases of sudden cardiac death?
CORONARY ARTERY DISEASE (CAD)
what is the mechanism that causes most cases of sudden cardiac death?
most often due to a lethal arrhythmia (asystole/V. f i b )
typical patient and predisposing factors for sudden cardiac death?
- younger victims
- factors:
- Hereditary or acquired abnormalities of cardiac conduction system/coronary arteries
- Myocarditis
- Cardiomyopathies
- Pulmonary hypertension
- Drugs
restrictive cardiomyopathy:
effect on cardiac function
Decrease in ventricular compliance –> impaired ventricular filling during diastole

restrictive cardiomyopathy:
pathogenesis
- Idiopathic, or
- Associated with systemic disease (radiation fibrosis, amyloidosis, sarcoidosis, metastatic tumors, inborn errors of metabolism), or
- Slightly enlarged
restrictive cardiomyopathy:
morphology
- not distinctive
- ventricles can be normal-sized, or slightly enlarged
- myocardium is firm and non-compliant
- bilateral dilation is common

what is: the deposition of extracellular proteins (insoluble beta-pleated sheets)
amyloidosis

pathogenesis of amyloidosis?
- due systemic disesae (myleoma), OR
- restricted to heart (senile cardiac amyloidosis)
what is amyloid composed of?
epidemiology of pts who carry mutation in this?
- transthyretin (amyloid is composed of transthyretin)
- 4% of african americans carry mutation in transthyretin
how to stain/visualize amyloidosis?
amorphous deposition –> can be seen w/ congo red & polarized light
–> apple-green birefringence
(think: “Amy Loid” eating an “Apple Green” in Congo Red?)

Organisms can cause direct injury or immune response against virally infected cells causes myocardial injury.
WHAT IS MOST COMMON CAUSE OF MYOCARDITIS IN US?
Viruses;
Coxsackievirus A & B
In addition to viruses, what were the other INFECTIVE causes of myocarditis emphasized in class?
- Bacteria (diphtheria toxin released by Corynebacterium causes injury)
- Lyme disease (self-limited conduction system disorder – may require pacemaker)
- Chagas disease (in S. America)
define: chagas disease
American trypanosomiasis, is a tropical parasitic disease caused by the protist Trypanosoma cruzi;
Sxs: an be mild, causing swelling and fever, or it can be long lasting.
Sequelae: Left untreated, it can cause congestive heart failure.
Myocarditis can result from Infective, Immune-Mediated Reactions, and Unknown causes:
What are the Immune-Mediated Reactions?
aka Hypersensitivity Myocarditis:
- Postviral
- Poststreptococcal (rheumatic fever)
- Systemic lupus erythematous
- Drug hypersensitivity (e.g. methyldopa, sulfonamides)
- Transplant rejection
Myocarditis can result from Infective, Immune-Mediated Reactions, and Unknown causes:
What are the Unknown causes (those that don’t fit under infected or immune mediated)?
- Sarcoidosis
- Giant Cell myocarditis
gross morphology of myocarditis
- Heart can appear normal, OR
- flabby with mural thrombi (advanced stages)
microscophic morphology of myocarditis
(NOTE: findings may be patchy and missed on biopsy)
- Edema
- Interstitial inflammatory infiltrate
- Lymphocytic infiltrate is most common
- Eosinophils in hypersensitivity myocarditis
- Myocyte injury (focal to extensive)

what are the microscopic morphology findings of Giant Cell myocarditis?
Multinucleated giant cells with focal or extensive necrosis (frequently extensive myocyte necrosis); aggressive!

which type of interstitial inflammatory infiltrate is found more commonly?
(lymphocytic or eosinophilic)
- Lymphocytic infiltrate is most common
- eosinophils in hypersensitivity myocarditis
microscopic morphology of myocarditis of Chagas disease?
Recall: Chagas disease is a tropical parasitic disease caused by the protist Trypanosoma cruzi;
On microscopy –> inflammatory cells and trypanosomes

Myocarditis clinical features can mimic which other pathology?
What are the clinical features of myocarditis?
- Myocarditis can mimic Acute Myocardial Infarction
- clinical features
- Spectrum from asymptomatic to precipitious onset of heart failure/arrhythmias
with sudden death - Fatigue
- Dyspnea
- Palpitations
- Fever
- Spectrum from asymptomatic to precipitious onset of heart failure/arrhythmias
What is a late complication of Myocarditis?
Dilated cardiomyopathy
(a condition in which the heart’s ability to pump blood is decreased because the heart’s main pumping chamber, the left ventricle, is enlarged and weakened. In some cases, it prevents the heart from relaxing and filling with blood as it should.)
how much fluid does the pericardial sac usually have?
<50 mL of thin, clear, straw-colored fluid
Differences b/w slowly accumulating and rapidly accumulating fluid in pericardial disease?
-
Slowly accumulating fluid –
- higher volume, 500 mL (sac has time to dilate)
- usually doesn’t interfere with cardiac function
-
Rapidly developing fluid –
- smaller volumes (200-300 mL)
- can be fatal (cardiac tamponade)
which type of Pericardial Effusion results from:
Congestive Heart Failure or Hypoalbuminemia?
Serous pericardial effusion
which type of Pericardial Effusion results from:
Blunt chest trauma, malignancy, ruptured MI, aortic dissection
Serosanguinous pericardial effusion
(contains or relates to both blood and the liquid part of blood (serum))
what causes CHYLOUS pericardial effusions?
Mediastinal lymphatic obstruction
Define: hemopericardium
Define: purulent pericarditis
- Hemopericardium: blood in the pericardial sac of the heart
- Purulent pericarditis: a localized infection within the pericardial space
Which types of Pericarditis are considered Chronic/Healed?
- Adhesive mediastinopericarditis
- Constrictive pericarditis
Which types of Pericarditis are considered Acute?
- Serous
- Fibrinous & serofibrinous
- Purulent/ suppurative
- Hemorrhagic
- Caseous
how does pericardial surface appear (gross anatomy) in acute pericarditis?
how does this change in chronic pericarditis?
- Acute –> Shaggy, irregular appearance of pericardial surface
- Chronic –> Heals with or without fibrosis

Type of exudate corresponding to following pericarditis:
- Acute viral
- Acute bacterial
- Acute hemorrhagic
- Acute viral – FIBRINOUS exudate
- Acute bacterial - FIBRINO- PURULENT exudate
- Acute hemorrhagic - BLOODY EFFUSION + FIBRINOUS exudate (often due to malignancy)
what are: delicate adhesions or dense, fibrotic scars that obliterate the pericardial space?
chronic pericarditis
what is: extreme case of chronic pericarditis wherein heart is completely encased in dense fibrosis and heart is unable to expand during diastole
Constrictive pericarditis

which types of cardiac tumors are most common?
Myxoma – most common primary heart tumor (benign)
- Metastatic tumors are more common
- Primary cardiac neoplasms are rare, most are benign
Myxoma:
location, effect on cardiac function
(recall: most common primary heart tumor)
- 90% are located in the atrium (more commonly in left atrium [fossa ovalis])
- Myxoma –> causes intermittent obstruction –> “ball-valve” obstruction –> **multiple syncopal episodes**
myxoma:
gross and microscopic appearance
- gross: gelatinous appearance
- microscopic:
- Stellate or multinucleated cells embedded in acid mucopolysaccharide ground substance

most common primary heart tumor in infants/children?
Rhabdomyomas
epidemiology of rhabdomyomas:
occurs in patients w/…, & course
- High frequency in patients with tuberous sclerosis (TSC1, TSC2 mutations)
- Course: Can regress spontaneously
what is the characteristic histology of RHABDOMYOMAS?
- “spider cells”
- large, rounded polygonal cells w/ glycogen-laden vacuoles separated by strands of cytoplasm

most common primary malignant heart tumor?
Angiosarcoma:
cancer that forms in the lining of blood vessels and lymph vessels. ‘
It often affects the skin and may appear as a bruise-like lesion that grows over time
What are: Abnormalities of the heart or the great vessels that are present at birth?
& epidemiology/percentage of all birth defects?
- CONGENITAL HEART DISEASE
- Epi: accounts for 20-30% of all birth defects; affects 1% of all newborns in the US
- higher rate in premature infants and stillborns
which congenital heart defects are more common in live births (versus stillborns)?
Defects involving single chamber/region of the hearts are typical in live births;
Surgical advances improve survival
most common congenital heart defect?
ventricular septal defect

pathogenesis of Congenital Heart Defects?
two overarching causes
- From: faulty embryogenesis during 3-8th week of gestation
- Causes:
-
environmental factors: congenital rubella infection, teratogens, maternal diabetes,
low folate -
genetic factors: (not one specific gene mutation)
- Single gene mutations (most involve transcription factors)
- Small chromosomal losses
- Additions/deletions of whole chromosomes (down syndrome, turner syndrome)
-
environmental factors: congenital rubella infection, teratogens, maternal diabetes,
most common genetic cause of congenital heart disease?
- **trisomy 13/15/18/ 21 (Down syndrome – most common genetic cause of congenital heart disease); due to additions/deletions of WHOLE chromosomes
- Most patients have no identifiable genetic risk & if genetic risk is identified severity of defect is highly variable
Major categories of congenital heart disease?
- LEFT-to-right shunt
- RIGHT-to-left shunt (cyanotic congenital heart disease)
- obstruction
define: shunt
define: cyanosis
shunt: abnormal communication b/w chambers/vessels
cyanosis: blue discoloration of skin
clinical features of LEFT-to-right shunt?
- Eisenmenge syndrome (LATE-ONSET cyanosis)
- Pulmonary hypertension
- Increased pulmonary resistance –> shunt reversal
- Right ventricular hypertrophy –> develops R–>L shunt –> cyanosis
define: Pulmonary hypertension
small pulmonary muscular arteries undergo medial hypertrophy and vasoconstriction
clinical features: Right-to-left shunt
- EARLY CYANOSIS
- Paradoxical embolism
- Clubbing of finger/toes (hypertrophic osteoarthropathy)
- Polycythemia

define: Paradoxical embolism
thrombi in peripheral veins BYPASS LUNGS and enter
systemic circulation
malformations associated w/ LEFT-to-right shunts;
which is most common form of congenital heart disease (CHD)?
-
**Ventricular septal defect (VSD) - *most common form of CHD
- Ventricular septum forms as fusion of muscular ridge (grows upward from apex)
with thin membranous partition (grows downward from endocardial cushions)
- Ventricular septum forms as fusion of muscular ridge (grows upward from apex)
-
Atrial septal defects (ASDs)
- Abnormal fixed opening in atrial septum (compared to PFO, septa are missing tissue rather than being unfused)
-
Patent ductus arteriosus (PDA)
- arises from left pulmonary artery and joins aorta distal to origin of left subclavian artery
In how many people does the patent formaen ovale remain?
20% of people;
- Flap covers foramen, but unsealed septa allows transient right-to-left blood flow
- (Valsalva maneuver during sneezing/straining)
- Paradoxical embolism
Atrial Septal Defects are usually well-tolerated and usually asymptomatic until age 30.
What is the most common ADS?
- 90% of ASDs are ostium secundum defects
- smooth-walled defect near foramen ovale
- usually w/o other cardiac abnormalities
- Others
- 5% are ostium primum defects
- 5% are sinus venosus defects

which type of Ventricular Septal Defects accounts for majority of VSDs?
Basal/membranous type accounts for 90% of all VSDs –> because this septum is last to develop
Note: 50% of small muscular VSDs close spontaneously
If pt has VSD, what else do you look for?
Why?
If pt has ventricular septal defect, pt likely has other congenital heart disease as well
(because only 20-30% of VSDs occur in isolation)
which type of congenital heart disease is associated w/ “machinery-like murmur”
patent ductus arteriosus;
90% of these are isolated defects
which malformations are associated w/ RIGHT-to-left shunt (aka cyanotic congenital heart disease?
which is more common?
- Te t r a l o g y o f F a l l o t ( TO F ) - most common cyanotic congenital heart disease
- Transposi t i on of t he Great Art eri es ( TGA)
cause and key features of tetralogy of fallot
Cause: anterosuperior displacement of infundibular septum
PROVe - key features
- P - Pulmonary stenosis (rigth ventricular outflow obstruction)
- R - Right ventricular hypertrophy
- O - Overriding aorta
- V - VSD (ventricular septal defect)

symptoms of Tetralogy of Fallot?
Symptoms depend on degree of pulmonary outflow obstruction/ subpulmonic stenosos:
**W/O SURGERY, MOST INFANTS DIE W/IN FIRST MONTHS OF LIFE
- baby feeding difficulties
- shortness of breath
- sudden deep blue discoloration (cyanosis)
- blue skin from poor circulation
- deformity of nails (clubbing)
- failure to thrive
Morphology of Tetralogy of Fallot
- (P) RV outflow obstruction – subpulmonic stenosis, PV stenosis, complete atresia of valve & proximal pulmonary arteries
- (R) Boot-shaped, enlarged heart (RVH)
- (O) Aortic valve lies over the VSD (overriding aorta)
- (V) VSD is typically large
- Dilated proximal aorta
- Hypoplastic pulmonary trunk
- Left side of heart is normal

what is: discordant connection of ventricles to vascular flow?
TRANSPOSITION OF GREAT ARTERIES
Aorta –> Right Ventricle
Pulmonary artery –> Left Ventricle
cause of transposition of great arteries;
what do patients rely on?
- Cause: abnormal formation of truncal and aortopulmonary septa
- Since (TGA) Separation of systemic and pulmonary circulations –> incompatible with postnatal life, patients rely on:
- VSD in 1/3 of cases
- PDA or PFO (structures will eventually close so babies need surgery right away to prevent closure)
morphology of TGA
- marked Right Ventricular hypertrophy
- hypoplastic Left Ventricle (underdeveloped, can’t effectively pump blood to body)
obstructive malformations:
which is most common OBSTRUCTIVE congenital heart disease?
- aortic coarctation: **most common obstructive congenital heart disease
- pulmonic valve stenosis
- aortic valve stenosis/atresia
aortic coarctation:
epidemiology, and types
- Epi: M>F, often in females w/ Turner syndrome
- Can be solitary defect, but >1/2 accompanied by bicuspid aortic valve
- Forms:
- Infantile form/ preductal
- Adult form/ postductal
differences b/w PREductal coarctation versus POSTductal coarctation?
- PREductal WITH a PDA
- sxs manifest early in life
- cyanosis localized in lower half of body
- infants won’t survive w/o surgical intervention
- POSTductal WITHOUT a PDA
- most asymptomatic
- RIB NOTCHING: due to collateral circulation developing in mammary and intercostal arteries–> enlarged –> creating notches in ribs
- HTN and strong pulses in upper extremities,
- Hypotension & weak pulses in lower extremities



