4: Ischemic heart disease Flashcards
2 key causes of Ischemic heart diease?
Which of the two accounts for the majority of the cases?
- Reduced Perfusion (*>90% of cases due to obstructive atherosclerotic lesions in coronary arteries)
- Increased myocardial demand
Which pathological cause of ischemic heart disease is associated w/ the following causes?
- Coronary emboli, myocardial vessel inflammation, spasm
- Hypoxemia, systemic hypotension
These cause REDUCED PERFUSION –> resulting in ischemic heart disease
(recall: >90% of cases due to obstructive atherosclerotic lesions in coronary arteries)
Which pathological cause of ischemic heart disease is associated w/ the following causes?
- Due to increased contractility, HR, ventricular wall tension/thickness (myocardial hypertrophy)
Due to increased myocardial demand
Percentage occlusion of coronary artery assoc with the 3 stages of ischemic disease?
- asymptomatic
- stable angina
- unstable angina
- asymptomatic - <70% occlusion
- stable angina - >70% occlusion
- unstable angina - >90% occlusion
four key clinical presentations of Ischemic heart disease?
- angina pectoris (chest pain)
- congestive hear failure
- myocardial infarction
- sudden cardiac death
definition: intermittent chest pain caused by transient, reversible myocardial ischemia
(15 sec-15 min)
angina pectoris
what is the pathological cause of Angina (chest pain)?
pain is a consequence of the ischemia-induced release of adenosine & bradykinin
type of ischemic heart disease assoc with the following description?
- Predictable chest pain associated with exertion
- Crushing/squeezing, substernal chest pain radiating down left arm/jaw
- Pain relieved by rest, drugs (nitoglycerin)
Typical/stable ischemic heart disease– most common
- reversible w/ medications (nitroglycerin)
type of ischemic heart disease assoc with the following description?
- Coronary artery spasm, can affect normal vessels
- Responds to vasodilators (relieved/reversible w/ medications)
Prinzmetal/variant – uncommon
(Responds to vasodilators (relieved/reversible w/ medications))
type of ischemic heart disease assoc with the following description?
- increasingly frequent pain, occurs at rest
Unstable ischemic heart disease
type of ischemic heart disease assoc with the following description?
- necrosis of heart muscle due to ischemia
- Epi: 10% occur <40 y/o; M>F
- Causes: atherosclerosis, vasculitis, amyloid, sickle cell disease
Myocardial infarction;
what is thought to cause “women being protected against MI during reproductive years?”
HORMONES are thought to have PROTECTIVE EFFECT
Describe the pathogenesis of myocardial infarction?
- Preexisting atherosclerotic occlusion
- New, superimposed thrombosis
- +/- vasospasm/ vasoconstriction
Preexisting atherosclerotic occlusion:
where does it occur?
-
LAD, LCX – first cm from aorta takeoff
- (left anterior descending)
- (left circumflex artery)
-
RCA – along entire length
- (right coronary artery)
- “critical stenosis” - if fibrous cap is eroded and ruptures –> completely occludes the lumen
- Collateral perfusion
define: critical stenosis
critical narrowing of an artery (stenosis) that results in a significant reduction in maximal flow capacity in a distal vascular bed
New, superimposed thrombus:
process of superimposed thrombsis
- Eroded/ruptured plaque
- Platelets adhere, aggregate, and are activated
- Coagulation is activated
+/- vasospasm / vasoconstriction:
describe this third stage of pathogenesis of ischemic heart disease
- Compromised lumen diameter
- Increases local shear forces –> further damaging the fibrous cap –> plaque disruption
review the acute plaque changes
- atherosclerosis –>
- plaque disruption –> healing –> severe fixed coronary obstruction (chronic ischemic heart disase)
- Plaque disruption –?
- mural thrombus w/ variable obstruction/ emboli
- occlusive thrombus

factors contributing to acute plaque change –> MI?
- vulnerable plaques
- adrenergic stimulation
*In the majority of cases, culprit lesions in myocardial infarction patients were NOT critically stenotic OR symptomatic before plaque rupture
(slide 11)
what are: large atheromatous cores or thin fibrous caps
VULNERABLE / UNSTABLE PLAQUES:
these are more prone to rupturing
how does adrenergic stimulation affect/contribute to acute plaque change?
- Adrenergic stimulation adds to plaque stress
- Causes of adrenergic stimulation:
- Surge in adrenergic stimulation associated with waking and rising
- Intense emotional stress
what are the 3 REVERSIBLE changes in the myocardial response to ischemia?
- Aerobic metabolism ceases ↓ATP ↑lactic acid
- Loss of contractility
- Ultra-structural changes on cellular level

what are the 2 irreversible changes in the Myocardial Response to Ischemia?
Irreversible changes occur in 20-40 minutes of prolonged ischemia
- Coagulative necrosis of myocytes
- Microvascular thrombosis

which aspect of the artery is MOST SUSCEPTIBLE to infarction?
SUBENDOCARDIAL ZONE is most susceptible;
susceptible to developing ischemia due to being FURTHEST AWAY from coronary artery
(endocardial zone is typically spared during periods of ischemia)

which type of infarcts involve full thickness of ventricle?
What is seen on ECG?
- TRANSMURAL INFARCTS involve full thickness of ventricle
- Yields ST segment elevations on ECG (aka STEMI)
Slide 14

which type of infarcts involve limited to inner third or myocardium?
What is seen on ECG?
- SUBENDOCARDIAL INFARCTS; limited to inner 1/3 or myocardium
- Typically show ST segment depressions (aka non-STEMI or NSTEMI
Slide 14

Left anterior descending (LAD) artery occlusion:
- what is it?
- where does it occur?
- what % of MIs?
- nickname for this occlusion?
- type of heart attack that’s caused by a 100% blockage of the left anterior descending (LAD) artery
- Location: Anterior LV wall, anterior 2/3rds of septum, apex
- 40-50% of MI; accounts for most of the causes of MIs
- “widow maker”

Right coronary artery (RCA) occlusion
- where does it occur?
- what % of MIs?
- Location: RV and posterior 1/3rd of septum and posterior LV (if PDA arises from
RCA) - 30-40% of MIs (2nd most common)
Left circumflex artery (LCX) occlusion:
- location
- % of MIs
- Lateral LV wall
- 15-20% of Myocardial Infarction

which artery perfuses 1/3rd of septum and posterior LV artery?
what’s its course?
Posterior descending artery;
- Arises from RCA (90%) or LCX –> dominant vessel
- PDA is often a branch off of Right Coronary Artery; but CAN be a branch
off of Left Coronary (Left Circumflex Artery) – termed “Left Dominant
Circulation”

how does the gross appearance of a myocardial infarct differ from hours:
- <4 hours
- 4-12 hours
-
<4 hours – no changes appreciated
- If preceded death by 2-3 hours, area can be highlighted by immersing tissue in triphenyltetrazolium chloride
- • 4-12 hours – occasional dark mottling (dark discoloration, on myocardium)
when do microscopic changes of myocardial infarct appear?
what are these changes?
- microscopic changes usually require >4 hrs
- changes:
- EM changes in first 4 hrs (sarcolemmal disruption, mitochondrial amorphous densities)
- Early coagulation necrosis
- Edema
- Hemorrhage
Slide 22

the image is of a triphenyltetrazolium chloride stain;
where is the ischemia?

Lateral Left Ventricle experienced ischemia:
due to Left Circumflex branch being occluded –> ischemia (can’t pick up the stain)
what are the gross findings of myocardial infarct at 12-24 HOURS?
Dark mottling (red-blue discoloration)
what are the microscopic findings of myocardial infarct at 12-24 HOURS?
- Coagulation necrosis
- Pyknotic nuclei (shrunken and dark)
- Myocyte hypereosinophilia
- Contraction band necrosis
- Early neutrophilic infiltrate

gross anatomical changes in a 24-72 hour myocardial infarct?
Mottling with yellow-tan infarct center
MICROSCOPIC anatomical changes in a 24-72 hour myocardial infarct?
- Coagulative necrosis
- Loss of myocyte nuclei
- Loss of muscle cross-striations
- Brisk neutrophilic infiltrate

gross anatomical changes of a 3-7 day old myocardial infarct?
- Gross changes are most prominent at 3-7 days
- Hyperemic border, central yellow-tan softening
- Central softening (feels gelatinous/ soft to touch), depression due to removal of dead tissue
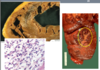
microscopic anatomical changes of a 3-7 day old myocardial infarct?
- Dead myofibers removed
- Neutrophils disappearing
- Macrophage infiltration

gross anatomical appearance of 7-14 day old myocardial infarct
- Maximally yellow-tan and soft (7-10 days)
- Red-gray (10-14 days)
microscopic anatomical appearance of 7-14 day old myocardial infarct?
- Granulation tissue at margins (7-10 days)
- 10-14 days:
- Well-established granulation tissue
- New blood vessel formation (angiogenesis)
- Collagen deposition (pink on histo slide is the new collagen being deposited)

gross and micoscopic anatomical features of 2-8 week old myocardial infarct?
- Gross: Gray-white scar
- Microscopic:
- Increased collagen deposition
- Decreased cellularity
- Macrophages are leaving the area

gross and micoscopic anatomical features of a >2 month old myocardial infarct?
- Gross - Scarring complete
- Microscopic - Dense collagenous scar
area is all scar tissue; nonfunctional tissue (can’t determine how old it is, but we know it’s older than 2 months)

what is REPERFUSION, and what can be done to stimulate this?
- Restoration of blood flow, which improves long and short-term survival
- **Goal is to reperfuse early**
- Thrombolysis (tissue plasminogen activator), angioplasty, or coronary arterial bypass graft
what is reperfusion injury?
what are the pathological changes that result locally upon reperfusion?
- Late restoration of blood flow to ischemic tissue can incite local damage
- Changes
- Mitochondrial dysfunction – mitochondrial contents are released and promotes apoptosis
- Myocyte hypercontracture
- Free radicals form and cause myocyte damage
- Leukocytes aggregate and may occlude microvasculature
- Platelets and complement are activated
how do hemorrhagic lesions occur after reperfusion injury?
- vasculature is injured during ischemia –> bleeding after flow is reestablished
- brown bc it’s experienced hemorrhage

JUST ONE CRUEL AND UNUSUAL HISTO QUESTION BC PROFS SUCKKK:
what does this histo image show?
- Contraction band necrosis – intensely eosinophilic stripes (closely-packed sarcomeres)
- dark pink vertical lines; buildup of calcium –> contraction bands form due to excess excitation (can’t relax, stuck in contracted state

how do we diagnose a myocardial infarction?
- clinical symptoms (**but 10-15% of pts may be asymptomatic)
- Severe, crushing substernal chest pain
- Radiation to neck, jaw, epigastrium, left arm
- Diaphoresis, nausea, vomiting
- labs
- EKG changes/ abnormalities
- STEMI and ST segment depression
what do irreversibly damaged myocytes release?
intracellular macromolecules
(such as Creatinine Kinase enzyme, Troponin free int he cytoplasm)

what are the 3 key biomarkers for myocardial ifnarctions?
- Which are most sensitive and specific?
- Which is best to identify subsequent MIs?
- Troponin I and T are MOST sensitive and specific
- CK-MB is more helpful to identify subsequent MIs

key consequences of myocardial infarction?
- Arrhythmias: risk for V. fib is greatest in first hour
- Mural thrombus: impaired contractility leads to stasis, endocardial damage creates thrombogenic surface
- Myocardial rupture
- Papillary muscle dysfunction –> post-infarct mitral regurgitation
- Pericarditis: 2nd or 3rd day after transmural infarct (full thickness of wall)
- Ventricular aneurysm: dilation/ballooning outward; can occur in the ventricle
myocardial rupture:
epidemiology, adn causes
- Epi: 1-5% of MIs and fatal
- Left ventricular free wall most common –> leads to hemopericardium, cardiac tamponade
- Occurs 3-7 days after infarction
- Ventricular septum –> leads to VSD
- Papillary muscle rupture –> leads to MR
the gross image is of what?

post-MI ventricular aneurysm;
Wall is dilated; there’s a dilation of the wall (myocytes have been
degraded by macrophages; –> so the area is more prone to formation
of aneurysm)
Chronic Ischemic Heart Disease:
- define?
- classic type of patient?
- Progressive heart failure secondary to ischemic myocardial damage – ischemic cardiomyopathy
- Patient:
-
Prior infarction(s) & compensatory mechanisms fail (hypertrophy of residual viable
myocytes) - Severe coronary artery disease (microinfarcts and replacement fibrosis without clinical evidence of a frank infarct)
-
Prior infarction(s) & compensatory mechanisms fail (hypertrophy of residual viable
Chronic ischemic heart disease:
morphology
- LV dilation and hypertrophy, areas of gray-white scars
- myocyte hypertrophy, diffuse subendocardial myocyte vacuolization, fibrosis
histological features in subendocardial zone after a myocardial infarct?
diffuse subendocardial myocyte vacuolization



