W3 - APs, nerve cells Flashcards
(37 cards)
Which components make up the nervous system?
Give rough numbers.
- neurons that are interconnected (5k - 200k times) to form neural circuits (1011 - 1012)
- neuroglial cells (1013)
- blood bessels, connective tissue
What are the functions of the nervous system?
- gathering of information, e.g. via receptors
- transmission of information
- processing of information, e..g memory, learning, behavior
List different type of neuroglial cells + their location and briefly explain their function.
in the CNS:
- astrocytes: regulate microenvironment, mediate entry of substances into CNS (K+, neurotransmitters), form glial scar after injury
- oligodendrocytes: one cell forms myelin sheath for many axons of diff. neurons
- microglia: latent phagocytes
- ependymal cells: specialized ependymal cells in choroid plexus secrete CSF, epithelial layer
in the PNS:
- Schwann cells: each cell forms myelin sheath for one section of the axon
- satellite cells: encapsulate dorsal root + cranial n. ganglion cells, regulate microenvironment

Explain the structure of neurons.
- dendrites: convey information to cell body, account for 90% of surface area, amount + shape dependent on type of neuron, well developed cytoskeleton
- cell body (perikaryon, soma): contains Nissl bodies (rER), prominent golgi, nucleus/nucleolus for protein synthesis
- axon: output of neuron, may have arborization (branches), arises in axon hillock (↑ Na+ channels, lacking organelels)
- axon terminal: forms presynaptic terminal

Which substances are transported by axonal transport?
Explain the process.
How fast is it?
motor proteins moving along the microtubule
- fast axonal transport for membrane bound organelles = 50-400 mm/d
- slow axonal transport for proteins = 1-10mm/d
either:
- anterograde w/ aid of kinesins: e.g. synaptic vesicles + enzymes resp. for synthesis of neurotransmitters
- retrograde w/ aid of dyneins: e.g. recycled synaptic vesicle membrane for lysosomal digestion
Explain the process of axonal degeneration.
Another name.
= Wallerian degeneration
- ER distends due to protein synthesis to repair destroyed axon
- ribosomes disorganized, soma swells, nucleus eccentric position, Nissl bodies stained weakly = chromatolysis
- in CNS: myealin sheath removed by phagocytosis
in PNS: Schwann cells undergo cell division

Explain the process of axonal regeneration in the PNS.
Why does it not happen in the CNS?
- axon ending sprouts
- ending elongates into path of Schwann cells
- reinnervate original peripheral target structure
happens at speed of ∽ 1 mm/d
in CNS axons also sprout, but oligodendrocytes do not form path, also formation of glial scar by astrocytes
What is the resting membrane potential?
potential difference between the intra and extracellular space when a cell is at rest (i.e., it is between action potentials, not performing any special function)
Values for ER of skeletal muscle and neurons.
- ER (sk. m.) = -90 mV
- ER (neuron) = -70 mV
What are pacemaker cells?
cells which’s EM is constantly changing, e.g. heart nodal cells
Explain the terms depolarization, repolarization, and hyperpolarization.
- depolarization: injection of positive charge, cell becomes more positive (e.g. -70 mV → -10 mV)
- repolarization: cell returns to ER
- hyperpolarization: injection of negative charge, cell becomes more negative (e.g. -70 mV → -80 mV)

What is diffusion potential?
potential difference generated across a membrane when an ion diffuses down its concentration gradient
BUT: equilibration → only transient
- magnitude depends on concentration gradient
- sign depends on charge of diffusing ion

What is equilibrium potential?
transmembrane voltage of a particular ion at which the influences of concentration gradient and electrical gradient on the ion’s movement exactly balance each other out
⇒ no net movement of the ion across the membrane

How can the equilibrium potential be calculated?
Nernst equation
describes EM when conc. on both sides of membrane are known (only 1 ion in solution)
Eion = -60/z * lg c1/c2
- z = no. of charges of ion
- c1, c2 = conc. of the 2 compartments
What are the equilibrium potentials of K+, Na+ and Cl- in skeletal muscle?
- K+: -94mV
- Na+: +65mV
- Cl-: -88mV
What are the equilibrium potentials of K+, Na+, Cl-, Ca2+ in neurons?
- K+: -90mV
- Na+: +60mV
- Cl-: -70mV
- Ca2+: +130mV
What is the membrane potential?
in a complex system w/ mulitple ions
permeant ions diffuse across the membrane down their concentration gradients (established by prim./sec. active transport mech.)
→ each ion “wants” to drive the Em toward its Eq
⇒ movement of most permeable ion will have greatest effect on Em
Which formula is used to calculate the resting membrane potential?
Goldmann-Hodgkin-Katz equation
also: chord-conductance equation
- pK = permeability constant for each ion
[Cl-]ic is on the bottom because its charge is negative
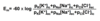
Which mechanisms contribute to the resting potential of a cell?
Values.
- diffusion potential of K+: modified by other ions, can be calculated w/ GHK → 90-95% of ER
- pump potential of Na+/K+-ATPase: electrogenic transport → 3-5% of ER<br></br> bc electrogenic (3Na+ out, 2K+ in, also: est. conc. gradient of K+)
- Donnan potential: bc of neg. charged proteins that remain in IC space → 2% of ER
Describe the effects of these events w/r/t the GHK (Goldmann-Hodgkin-Katz equation):
- the opening of K+ channels
- the opening of Na+ channels
- the opening of Cl- channels
pK resembles opening/closure of ion channels for that particular ion
opening of K+ channels:
↑[K+]EC → entire ratio ↓ → Em becomes more positive = hyperpolarization
opening of Na+ channels:
↑pK[Na+] → pK[K+] and pK[Cl-], both negligible → Em close to ENa+ = overshooting depolarization
opening of Cl- channels:
↑pK[Cl-] → pK[K+] and pK[Na+], both negligible → Em close to ECl- = stabilization of ER (bc ER ∽ ECl-)
all assumptions refer only to the general cell behavior

Describe the structure of Na+ and K+ channels.
Na+ channels:
- β1, β2 subunit
- α subunit: 4 six transmembrane helices that form the wall of the pore
- voltage-gated (activation gate + inactivation gate)
K+ channels:
- 4 six-transmembrane helices
- voltage-gated (1 gate)
Which substances are able to block Na+ channels?
Which substances are able to block K+ channels?
Na+ channel blockers:
- tetrodotoxin (TTX) from extracellular side (in ovaries of puffer fish)
- lidocaine (local anesthetic)
K+ channel blockers:
- tetraethylammonium (TEA+) from cytoplasmic side
Which mechanism is used by ion channels to cause non-specificity?
selectivity filter
ions can pass through pore depending on size of hydration shell surrounding the ion
e.g. only K+, no Na+
How is Ohm’s law applied to describe the electrochemical gradient?
Ohm’s law: V = R * I
⇒ Iion = gion (Em - Eion)
- V = Em - Eion
- 1/R = g = conductance, permeability









