Unit 6 - Apoptosis, advanced therapies and concepts of immuno-oncology Flashcards
4 reasons why cells die
- when they get old e.g. lifespan of RBC = 120 days
- irreversible damage - exposed to extensive damage e.g. ischaemia, stress e.g. pathophysiological conditions, fever
- when they become superfluous - tissue/organ development e.g. loss of interdigital web cells, loss of tadpole tail; elimination of superfluous immune cells after recovery from infectious disease
- when they become dangerous to the body/organism - virus infected cells, malignant/cancer cells
how do cells die
apoptosis - highly regulated, organised form of cell death
necrosis - dysregulated form of cell death
characteristics of apoptotic cells
programmed form of cell death
cell separates from neighbouring cells
cell shrinkage
membrane blebbing
chromatin condenses and the nucleus fragments
cell breaks up into apoptotic bodies which are phagocytosed, such that cellular components or waste products do not produce an inflammatory response
predictable, reproducible sequence of events

key differences between apoptosis and necrosis
APOPTOSIS
- single cells die
- cell shrinks
- remnants are phagocytosed
- neat, controlled
- no trace left
- no inflammation follows
NECROSIS
- chunks of tissue die
- cells swell and burst
- remnants are not cleared
- cell content released into EC space
- inflammation follows

too little apoptosis
malignant and pre-malignant conditions
lymphoproliferative disorders
leukemias
lymphomas
solid tumours
too much apoptosis
alzheimer’s
parkinson’s
stroke
atherosclerosis (CV)
ischaemia/reperfusion injury (CV)
dysentery (intestinal)
diarrhea (intestinal)
phases of apoptosis
INITIATION
the cell makes the decision to kill itself
EXECUTION
cell commits itself to die and activates the machinery for cellular disassembly
CLEARANCE
the apoptotic cell/bodies are removed from the system
what initiates apoptosis
appearance of death signals
withdrawal of survival factors
categories of death signals that initiate apoptosis
EXTRINSIC
death signal can derive from environment of cell
hormones and cytokines e.g. death ligands secreted by immune cells can kill infected/cancerous cells
INTRINSIC
can derive from inside of cell
overwhelming stress/irreparable damage (DNA damage, hyperthermia, exposure to toxic compounds)
withdrawal of survival factors - initiate apoptosis
most cells in the body depend on the presence of growth factors
in their absence, apoptosis is initiated and cells die
e.g. immune cells depend on presence of IL e.g. T cells on IL-2 and IL-15
what are caspases
effectors of apoptosis
cysteine dependent aspartic acid specific proteases
co-ordinate destruction of cellular structures
hallmark of apoptosis - required
proteases
enzymes that catalyse the breakdown of proteins into smaller polypeptides or AAs
cysteine dependent
active site of the caspase contains cysteine residue that is required for its catalytic activity
aspartic acid specific
cleave substrate proteins at aspartic acid residues
how are caspases synthesised
as inactive precursors (pro-caspases)
2 groups of caspases
initiator - 8, 9, 10
effector - 3, 6, 7
initiator activate effector, which then mediate apoptosis through the proteolytic cleavage of 1000s of proteins
initiator caspases
8
9
10
effector caspases
3
6
7
extrinsic activation pathway is also known as
death receptor mediated pathway
intrinsic activation pathway is also known as
mitochondrial mediated pathway
death receptors involved in extrinsic pathway
when are they expressed
family involved
subset of TNFR superfamily - TNFR1, Fas, TRAIL-R1, TRAIL-R2
some receptors are constantly present on cell surface, while other are expressed only upon damage
external cysteine rich domain - involved in ligand binding
transmembrane domain
internal death domain (DD) - needed for binding of adapter proteins like FADD
MOA of extrinsic pathway
death receptors e.g. TNFR, FasR are transmembrane receptors present on cell surface
binding by death ligands e.g. Fas causes death receptor to oligomerise
death receptors change shape and recruit adaptor molecules e.g. FADD or TRADD
several pro-caspase-8 molecules recruited and transactivate each other
active caspase-8 (initiator caspase) cleaves other caspases promoting irreversible cascade and cell death
DISC - Death Inducing Silencing Complex

intrinsic cell death pathway
when is it activated
key event
family involved
activated in response to a variety of internal stresses including DNA damage, ER stress, growth factor deprivation
release of mitochondrial intermembrane space proteins is the key event in intrinsic cell death
mitochondrial mediated release of intermembrane space proteins is controlled by the BCL-2 family
BCL-2 family
function
what do they have
central controllers of intrinsic cell death
BCL-2 family members have at least 1 of 4 conserved motifs
bcl-2 homology 1 - 4’ BH1, BH2, BH3, BH4
important in regulating interaction between family members

3 broad classes of BCL2 family members
pro-apoptotic effector proteins - BAX and BAK
anti-apoptotic protein - BCL2, BCL-XL, MCL1
pro-apoptotic BH3-only proteins - BID, BAM, BIM, NOXA
balance between pro and anti apoptotic BCL-2 family members determines cell fate
pro-apoptotic effector proteins
BAX
BAK
anti-apoptotic proteins
BCL2
BCL-XL
MCL1
pro-apoptotic BH3 only proteins
BID
BAD
BIM
NOXA
overview of intrinsic pathway

apoptosis in cancer
tumour cells under great stress
DNA damage, lack of nutrients, lack of O2
cancer therapies normally induce apoptosis

imbalance of apoptosis
autoimmune diseases - Lupus, rheumatoid arthritis, type 1 diabetes
AIDS, CD4+ lymphocyte depletion
modified expresson of apoptotic pathway proteins - cancer
increased expression of anti-apoptotic BCL2 family members
elevated XIAP expression (intrinsic)
Bax, TSG mutated in some colon tumours
p53 is a TSG down regulated in many cancers
destruction of pro-apoptotic proteins
proteosome degrades proteins and can be over-active in certain cancers
selective BCL-2 inhibitors could treat
chronic lymphocytic leukemia
acute myeloid leukemia…
what induces apoptosis in cancer cells through the extrinsic pathway
cell-mediated immunotherapy
immune-checkpoint inhibition
cells of the immune system

most abundant leukocyte
what do they do
neutrophils are by far the most abundant leukocyte circulating - comprise > 50% of leukocytes
adept at phagocytosing and killing microbes

myeloid cells
neutrophil
eosinophil
basophil
immature DC
mast cell precursor
monocyte → macrophage
platelet
erythrocyte

macrophages and dendritic cells - function
detecting and instigating immune responses
presenting the components of phagocytosed microbes to cells of the lymphoid system
escalating immune responses through the secretion of multiple cytokines and chemokines
macrophage

dendritic cell

lymphoid cells
function
T and B lymphocytes
adaptive immune system
can generate highly specific cell surface receptors through genetic recombination of a relatively limited number of precursors for these receptors
T cell receptors = TCRs
B cell receptors = antibodies
both can be highly specific for particular molecular structures (ANTIGENS)

lymphoid cells
B-cell
T-cell
NK
(mature DC)

NK cells function
innate immune system
police presence of special antigen-presenting molecules (MHC I)
use germline-encoded receptors (NK receptors) that are distinct from the receptors of T and B cells and are endowed with the ability to kill cells that express abnormal MHC receptor profiles, as well as stress induced molecules
T cells recognise infected or tumour cells in an antigen dependent manner
NK cells recognise infected or tumour cells in an antigen independent manner (no need for antigen exposure or immunological memory)
distinguish between T cells and NK cells
T cells recognise infected cells in antigen dependent manner
NK cells recognise infected cells in antigen independent manner
how do the innate and adaptive immune systems work in tandem
infection occurs → innate serves as a rapid rxn force that deploys a range of relatively non-specific weapons to eradicate the infectious agent or at the very least to keep the infection contained
gives time for initially sluggish adaptive immune system to select and clonally expand lymphocytes with receptors (TCR and antibodies) that are capable of making a much more specific response that is uniquely tailored to the infectious agent - adds new weapons to the attack
role of macrophage
detect infectious agents - an array of pathogen recognition receptors (PRRs) borne on their plasma membranes e.g. Toll-like receptors as well as other cellular components such as endosomes
DON’T RECOGNISE ANTIGENS, instead:
Pathogen Associated Molecular Patterns (PAMPs)
Danger Associated Molecular Patterns (DAMPs)
examples include certain sugars not seen in humans e.g. lipopolysaccharide (LPS/endotoxin - cell wall of bacteria), nucleic acids
dying cells also release factors capable of activating PRRs
how does the macrophage work
- macrophage is put on a state of high alert (activated) and is now better at engulfing (phagocytosis) and killing any microorganisms it encounters
- macrophage begins to secrete cytokines and chemokines, which enhance vascular permeability and attract other immune cells to cause inflammatory response

macrophage activation

tissue macrophages initiating immune response to local bacterial skin infection

neutrophil extravasion into tissues
cytokines produced by macrophages induce expression of receptors on endothelial cells (selectins) that bind to ligands on neutrophils causing them to decelerate on the BV wall
chemical attractants (chemokines) attract into tissues - WBCs slow down when interacting with these - sense chemokines - chemicals released under tissue - activation of molecules on neutrophil - attaches to endothelial lining and then migrates out - into deeper tissues where inflammatory stimulus is coming from
migration is facilitated by strong adhesion molecules on the neutrophils - integrins

what do NK have the ability to do
role of MHC
inspect host cells for signs of abnormal patterns of protein expression that may indicate that such cells might be harboring a virus - immunosurveillance
capable of killing cells that have suffered mutations and are on the way to malignant transformation into tumours
health cells express MHC molecules - NK cells have inhibitory receptors which recognise MHC - if cells lose MHC, they are vulnerable to killing by NK cells (missing self)
predominant mode of killing = release of cytotoxic granules containing lytic proteases Perforin and Granzyme
NK cells also release cytokines to activate immune response
genetic instability (as a result of cancer) and/or viral infection can lead to expression of protein ligands that are recognised by activating receptors on NK cells

apart from MHCs, what else do NK cells recognise
antibody coated cells via their Fc receptors, targeting them for destruction (antigen dependent cytotoxicity)
B cells are the source of antibodies
once activated, NK cells can also kill target cells via expression of death inducing ligands, which induce apoptosis (FAS or TRAIL)

how do NK cells co-operate with macrophages
cytokines produced by macrophages can activate NK cells
NK cells can recognise PAMPs and DAMPs e.g. nucleic acids from dying cells
cytokines released by activated NK cells important for maturation of dendritic cells and in turn enhance macrophage function

how do NK cells form a bridge between innate and adaptive immunity
when NK cells kill cancer cells, the antigens released are taken up by antigen presenting cells - dendritic cells
cytokines produced by NK cells stimulate maturation of dendritic cells
mature dendritic cells present tumour antigens in association with their MHC molecules to helper (CD4) and cytotoxic (CD8) T cells
CD8 cytotoxic cells can then recognise and kill antigen expressing tumour cells
CD4 T cells help B cells produce antibodies against these antigens
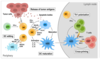
cells that present antigens
dendritic cells
where are DCs produced
how did they get their name
DCs are produced primarily in the bone marrow and derive their name from the multiple long membrane projections or dendrites that these cells possess
they share a common progenitor with macrophages
both macrophages and DCs have somewhat overlapping functions
cells responsible for adaptive/acquired immune response
mediated by lymphocytes
→ T lymphocytes (T cells)
→ B lymphocytes (B cells)
what characteristic do T and B cells possess
defining characteristics of the acquired immune response
both highly antigen specific
both exhibit immunological memory whereby they respond more vigorously upon re-encounter with specific antigen
where do T cells develop
from bone marrow precursors in the thymus
3 major functions carried out by T lymphocytes
- providing assistance to other cells in the immune response - helper T cells
- Limiting excessive or undesired immune responses – regulatory T cells
- killing cells infecting with pathogens - cytotoxic T cells
function of B cells
develop fully within bone marrow
produce antibodies - humoral immunity
response that recognises antigens
specific acquired immune response
shape of antigen
3-D shape that is complementary to antibody molecules that act as the antigen receptor on B lymphocytes
antigen-specific antibody molecules are subsequently released in a soluble (secreted) version by plasma cells derived from B cells following their activation
antigens can be proteins, CHOs, lipids, nucleic acids, small molecules
apart from B cells, what other cells recognise antigens
T cells
usually in form of proteins that are digested from the original polypeptide into short peptides
peptides are then shown to the antigen receptor on the surface of T cells using a MHC which is specialised to show peptides to the T cell receptor (TCR)
T cell therefore recognises a shape that is a combination of antigen-derived peptide and MHC
structure of antibody
variable region - devoted to binding to the individual antigen (antigen recognition function)
constant region - concerned with linking to the complement, phagocytes (e.g. macrophages) and NK cells (effector function)
body must make millions of antibody molecules with different antigen-recognition sites but that all share the property of recruiting other elements of the immune response

Antibodies and Effector Cells (7 mins)
ADCC = Antigen Dependent Cellular Cytotoxicity
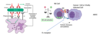
B cell activation and antibody production
when the antigen binds to a complementary B cell receptor - a complex containing an antibody on the surface of the B cells → activating signal leading to increased proliferation and survival of B cell

normal antibody production
where does it take place
MOA

takes place in germline centre of lymph node where Follicular Dendritic Cells present antigen to the B cells
only those cells that receive the strongest survival signal due to the best antigen-antibody fit are selected and survive - others undergo apoptosis
further help from helper T cells enable the selected B cells to mature into either anitbody producing plasma cells or memory B cells, which are primed waiting to mount a rapid antibody response in case the antigen is encountered in the future

what is the MHC
how are MHCs recognised
structure
genetic locus that control histocompatability
in early transplantation experiments, the donor and recipient were found to need the same MHC locus in order to avoid graft rejection
MHC molecules are recognised by T cells via TCRs
MHC = polygenic and polymorphic
3 regions = class I (contains genes encoding 6 MHC proteins) and class II (variable number of genes that ultimately encode 3 heterodimeric MHC proteins)
MHC genes are highly polymorphic with 1000s of different alleles within population
MHC antigens in humans = HLA antigens (human leukocyte antigens) as they were first identified on WBCs
MHC antigens =
HLA antigens
how does the TCR work
T cells express TCRs - recognise antigens being presented by MHC molecules
CD8 + T cells recognised internally derived antigen being presented on MHC class I molecules allowing CD8 + T cells to scan cells for internal threats
CD4 + T cells recognise endocytosed antigen presented on MHC class II molecules of professional anitgen-presenting cells, thereby allowing recognition of EC antigens
structure of TCR
highly variable
heterodimer consisting of either α and β OR γ and δ chains
these chains undergo random rearrangement at the genetic level to generate a wide variety of TCRs

what do most T cells express
αβ TCRs with which they recognise their antigen being presented by MHC
how does the TCR signal
forms a complex with CD3 molecules
TCR itself has a short cytoplasmic tail with no signalling capability
it forms a complex with CD3γ, δ, ε and ζ chains
the cytoplasmic tail of CD3ζ contains an immunoreceptor tyrosine-based activation motif (ITAM), which is phosphorylated, allowing it to signal
what does the cytoplasmic tail of CD3ζ contain
immunoreceptor tyrosine-based activation motif (ITAM), which is phosphorylated, allowing it to signal
what is the TCR similar to
how do they differ
similar to Fab fragments of B-cell receptors/antibodies
both composed of 2 different peptide chains and have variable regions for binding antigen, constant regions and hinge regions
principal differences - TCRs remain membrane bound and contain only a single antigen-binding site

T cell receptor activation
TCR is assisted by CD4 or CD8 receptors
recognises peptide antigen in context of MHC molecules
TCR activation signals are propogated via the CD3 co-receptor complex, which is made up of CD3 γ, δ, ε and ζ chains
co-clustering of CD4 or CD8 with the TCR complex facilitates signal propogation through phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) with the CD3 ζ chain
T cell adhesion and activation
initial stable interaction between APCs or target cells and T cells following antigen recognition is facilitated by adhesion molecules, which causes cells to stick more tightly together
in addition to antigen recognition via TCR along with CD3 signalling, T cells require a 2nd activating signal (signal 2) to fully activate and avoid anergy (unresponsive) or apoptosis
co-stimulatory molecules on APC’s bind to CD28 and this provides this 2nd signal
by upregulating an anti-apoptotic protein Bcl-xL, CD28 stimulation blocks TCR-mediated signals that would otherwise result in apoptosis (activation-induced cell death)

what do cytokines do to signal
provide signal 3 to promote clonal expansion and proliferation
further stimulation and activation (signal 3) can be provided by cytokines e.g. IL 2 (released by dendritic cells or macrophages)

T cell adhesion and activation
initial stable interaction between APCs or target cells and T lymphocytes following antigen recognition is facilitated by adhesion molecules, which cause cells to stick more tightly together

killing by cytotoxic lymphocytes and NK cells
NB NK cells - innate killers
Antigen independent
Recognise virally infected cell or tumour cell because it has
- Lost MHC molecule
- Upregulated stress molecules - danger associated molecular patterns - ABNORMAL
Adaptive response - B cells produce antibodies - NK cells may further contribute to ongoing immune response through ADCC
Virally Infected or cancer cells - sometimes downregulate MHC class I so T cells no longer recognise them but this then makes them vulnerable to killing by NK cells

controlling T cell activation
uncontrolled T cell activation could be very harmful and lead to autoimmune disease
dampening down of T cell responses occurs via a number of mechanisms, some of which operate at the level of the actvated T cell itself, while others operate via additional T cell subsets - regulatory T cells
molecules present on activated T cells that serve as “off switches” for such T cells represent important immunological checkpoints, helping to keep T cell responses within certain limits
immune checkpoint receptors and their ligands
immune checkpoint receptors - PD-1 and CTLA4
ligands - PD-L1 and CD80/86
T cells expressing these molecules are suppressed - molecules are ligated by PD L1
Propogation of inhibitory signals in T cell that turns off T cell cycle - no further cytotoxic T cell activation
Important in normal homeostasis
in cancer this is exploited by tumour - T cell exhaustion

immune checkpoints and activation induced cell death
signalling via TCR and CD28 leads to T cell activation
inhibitory receptors or “immune checkpoints” e.g. CTLA-4 and PD-1 are upreg. on activated T cells, usually after prolonged activation in the presence of their ligands on APC’s or tumour cells can induce T cell inhibition
activated T cells upregulate Fas receptor
in absence of co-stimulation by CD28 Fas ligand expressing cells can induce apoptosis (activation induced cell death)
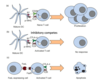
regulatory T cells
how do they work
Tregs are able to suppress T cell activation by expressing ligands for immune checkpoints, releasing suppressive cytokines or eliminating effector CD8/CD4 T cells
Tregs are important in preventing autoreactivity (autoimmune disease)
in cancer, Tregs are often over-active, leading to an impaired immune response

what is the immune system designed for
how does cancer pose a challenge
immune system has evolved to deal with infection (non-self antigens) rather than cancer (altered self)
recognising and mounting a robust immune response against mutated cancer cells that may only have subtle differences from normal cells is challenging
mutational processes associated with the development of cancer frequently generates neoantigens that, in principle, can elicit T cell responses, in practice such responses are highly muted because of mechanisms that serve to prevent the emergence of autoimmunity
how does cancer manipulate the immune system
well-meaning regulatory T cell responses and other mechanisms that serve to limit the development of autoimmunity (such as CTLA-4 and PD-1 mediated downregulation of T cell responses) conspire to suppress the immune response against cancer
tumours also actively manipulate the immune system to minimise immune responses that do emerge
tumours recruit macrophages, neutrophils and other innate immune cells and “re-educate” such cells towards a wound-healing phenotype for the purposes of supporting tumour growth and survival
“cancer = wound that does not heal”
cancer progression over time

immune editing
gradual stepwise development of tumours over long periods of time permits the selection of cells that are effectively invisible to the immune system - must avoid killing by immune system
if they are not, such cells are weeded out by the immune system as the tumour develops
this process selects for the “fittest” tumours that are very difficult for the immune system to deal with

how can the immune system become tolerant of tumour antigens
in the absence of sufficient activation
Cytotoxic T cells can’t deal with cancer
Antigens taken up by dendritic cells
Co-stim. signals
Proper functioning T cells
If dendritic cells take up antigen - various cytokines
T cells - recognised antigen but cannot mount response

ways in which cancer cells can evade or suppress the immune system
loss of MHC expression by cancer cells means T cells unable to recognise tumour antigen
however this can make them vulnerable to NK cells
expression of certain ligands can either suppress (e.g. PD-L1) or kill (Fas Ligand) immune cells

T cell checkpoint inhibition
how does it work
what are the molecules involved
T cell checkpoint molecules (CTLA-4, PD-1) are frequently engaged by tumours to suppress anti-tumour T cell responses
cancer cells frequently engage immune checkpoint molecules on T cells, such as PD-1 and CTLA-4, which has the effect of anergizing T cells in the tumour environment
blocking antibodies against CTLA-4, PD-1 and PD-L1 can reactivate tumour-specific T cells and can now play a role in treatment of solid cancers

7 approaches to cancer immunotherapy
- passive immunotherapy with monoclonal antibodies
- unmasking of latent T cell responses by targeting immune checkpoint molecules
- antigen-independent cytokine therapy
- vaccination approaches to stimulate immune responses against tumour antigens or the tumour vasculature
- adoptive transfer of ex vivo expanded T, NK or DCs
- adoptive transfer of ex vivo generated chimeric antigen receptor (CAR) T cells
- bispecific T cell engagers
approved monoclonal antibodies

approved monoclonal antibodies

MOA - monoclonal antibodies
Recognition by NK via Fc receptor
NK recognises Fc - release cytotoxic granules - perforin forms pores in tumour cells to allow other contents in - lysis
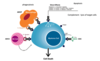
CD20 monoclonal antibodies
CD20 = antigen present on mature B cells
when CD20 antibodies bind to CD20 antigens on surface of malignant B cells it can induce cell death by:
fixing complement leading to cell lysis
direct apoptotic signals
ADCC (NK cells)
phagocytosis (macrophages bind antibody via Fc receptors)

therapeutic use - how to reactivate cytotoxic CD8 lymphocytes
CTLA-4, PD-1 and PD-L1 blocking antibodies

retuxemab
CD20 antibody
non-hodgkins lymphoma
chronic lymphocytic leukemia
CAR T cell therapy
acute lymphoblastic leukemia
no MHC needed
chimeric antigen receptors

chimeric antigen receptors - MOA
In normal t cell receptor CD28 would be needed for co stim. But all built in car t cell molecule - recognises tumour cell antigen even in absence of MHC - sufficient to activate killing by T cell

CAR-T procedure
HIV - retroviruses - good at stable genetic modification
Leukapheresis - T cells extracted and we put in retrovirus that can genetically express
Need cytokines to proliferate and expand
After chemotherapy (done to get rid of lymphocytes)

challenges of CARs

what ensures survival of MM cells
BCMA
represents a good target for immunotherapy
- Can recognise Tumour antigen (BCMA)
- Other arm - recognises CD3
- Brings t cell to close proximity to target cell and leads to recognition of tumour cell by t cell - recognition and release

options other than monoclonal antibodies for targeting Myeloma antigens
CAR T cells
bispecific T cell engagers
bispecific antibodies
antibody-drug conjugates



