Unit 3-Nuclear Chemistry Flashcards
Most atoms have a stable nucleus which doesn’t change.
However, some isotopes (radioisotopes) are unstable and undergo changes in the nuclei?
What is this called?
Radioactive decay
Radioactive elements can become stable by giving out which 3 types of radiation?
Alpha, beta and gamma
What are the electrical charges of Alpha, Beta and Gamma radiation?
(Positive, no charge or negative)
Alpha-Positive
Beta-Negative
Gamma-No charge
Radiation can penetrate objects of different thicknesses.
Which is the most and least penetrating radiation?
Alpha has a mass of 4 and so is least penetrating.
Gamma is most penetrating.
When writing nuclear equations, how are alpha and beta particles represented?

State the equation for when Thorium 232 loses an alpha particle.
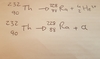
State the equation for when Carbon 13 loses a beta particle.
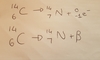
What is meant by the half-life of a radioisotope?
The half-life of a radioisotope is the time taken for the sample’s activity to fall by half
or
Half-life is the time for half of the nuclei of a particular isotope to decay.
The mass of a radioisotope falls from 3.2g to 0.4g in 2 hours.
What is the half-life of this radioisotope?
- How many times has 3.2g been halved to get to 0.2g
- 2g→1.6g→0.8g→0.4g→0.2g
Four half-lives
- 4 half-lives = 2 hours
1 half-life = 0.5 hours (30 minutes)
The half-life of a source is 8 days.
Calculate the fraction of the source that would remain after 16 days.
At the start, there would be 100% of the sources.
After 8 days there would be 50% (1/2) left.
After 16 days there would be 25% (1/4) left.


