Thermodynamics Flashcards
thermal energy
Total KE of particles
Thermal enegy depends on how large you are, total KE of all particles
Two objects can have same temperature but not the same energy, if i am bigger have more particles so overall have more thermal energy because more particles so ottal energy bigger even if at same temp
One thing can have a higher temperature but less overall thermal energy, why becuase depends on the size, if I am very big may be at a lower temperature but could have more overal KE b/c I am so large
Also means can be at a lower temp and have more thermal energy becuause if I am big enough total amount of thermal energy I have could be bigger than total maount of thermal energy you have even if at higher temp
Like comparing density and mass, temp like density doesn’t matter how much you have like an average; thermal energy liek mass the bigger you are the more you will have
internal energy
total KE and PE of particles, all the energy they have of each other!
if add heat temp goes up aveage KE rising, heat energy is going into KE of particles, but then other sections on heating curve where temp constant where is that energy going then?
Has to be going somewhere else, changes phase separates particles and that changes energy, if heat energy flowing in makes sense for internal energy to rise, energy has to go somewhere* BUT oesnt have to go to PE can make substance hotter, can go through changing phases
Heat
transfer of thermal energy from one thing to another
what does that mean?
heat transfer is redundant, heat means the transfer it means that the thermal energy is flowing from one thing to another thing, just the way we talk about it.
HEAT is a process** A FLOW you cannot “have” heat* heat can flow into you raising temp or internal energy, but you do not have heat**
temperature
measures average KE of particles
measures not just KE but TEMP MEASURES AVERAGE KE in substance
as KE inc temp inc
how is heat transfered? how is energy transfere what are mechanisms of heat transfer?
3 types
- conduction
- convection
- radiation
firrs two require a mediuM! for stuff to be in contact has to be actual stuff
but radiation is electromagnetic waves, this can travel through a vacuum***= SO ONLY WAY TO TRANSFER HEAT ENERGY ACROSS A VACUUM***
conduction
collision in one substance collides with particles in another subtance transferring some of their energy, stuff touching so therefore we can have this transfer by particles of one thing colliding with particles of another thing and then some of the energy is transfered
convection
conduction through a fluid
this means will have some hot place and some other colder place, and in between them is a fluid of some kind, the hot thing heats the fluid via conduction, then the fluid particles move around and then teh fluid particles will heat the cooler thing* classic exampel of this is an oven, in an oven we have hte heating element very hot; heats the air which is a fluid, then the air that fluid is responsible for heating the fluid that is convection
radiation
transfer of heat energy via electromagnetic waves of photons*
all objects with temperature emits photons
so what happens here- if I am hotter than my surroundings I will end up releasing more energy via photons than surroundings, if i am cooler than surroundings i am emitting photons surroundign emitting photons but I am doing less than they are so I get warmth*
so transfer of photons between objects
why metal coffee cups work
which is why our best thermal insulator is a vacuum** becuase it prevents conduction adn vonvection completely* if think about those coffee cups metal which is a good conductor of heat, how can I make ea cup out of metal and expect that ot insulate? that should not be possible? doesn’t work so what is insulatig the cup? those cubs we are taling about have 2 walls, is a vacuum, way they make these cubs make this hting suck all air out between walls, so best insulator can get is having nothing in there becuase it prevents conduction and conention, doesnt matter if metal good conductor, no conducto to conduct heat from inside of cup to otuside of cup barrier layer of nothing
like sun, all heat energy transfered via radiation via photons
adiabatic
refers to a process in which there is NO HEAT TRANSFER***
NONE
- But there is always radiative heat loss
- so how we do this is by doing it quickly, faster you do it less opprotunity there is for any mechanisms of heat transfer to happen they all take time!
- ALL TYPES OF HEAT TRANSFER TAKE TIME, if you do the thing really really quickly no time for particles to collide and transfer energy or a significant amount of their energy so the faster you do something the closer you will get to adiabatic
best thermal insulator:
VACUUM
prevents convection and conduction
vacuum does not prevent radiation* if relying on physical insulator or vacuum cannot prevent radiation**
2nd law of thermodynamics
- universe just geting shitter
- change in entropy of universe FOR ANY PROCESS is always positive or equal to zero, it is impossible to decrese the entropy of the universe as a whole, the universe is ALWAYS getting more disordered
- S= entropy, disorder
- things are always getting worst, and more disorder
- change S greater or equal to 0
1st law of thermodynamics
Conservation of energy
∆E = Q – W
∆E = change in internal energy of a system
Q= HEAT that flows in or out
W=work done by the system
Why is this a conservation of energy statement? Key thing to understand Q and W are ways of transfering energy, Q is the flow of thermal energy W is mechanical work; so what this statement is relly saying can only change energy of soething if put energy in our take energy out of the system, otherwise the total energy of the system should remain the same* that is why this is a conservation of energy statement*
For heat
and for work
analyzing first law of thermodynamics
for heat:
Q>0 =when heat flows in - makes sense if process is endothermic and energy flwoign in makes sense to us and delta h is positive
Q<0
=if Q is going out, say Q is negative like our exothermic thing
for work:
w>0 if I am doign something or puttin gin effort, means work is positive system is trying to do something but the internal energy of the system should go down, work is positive when the system does work on the surroundings* so when system does work on surroundings system is posistive, using energy to push what is around it, means its internal energy that goes down makes sense, minus a positive becomes negative; ENERGY GOES DOWN
if work is neg, W<0 means surroundigns do work on system, pushing in on system giving system more energy ; negative value means internal energy goes up, becuase minus a negative is a psoitive; ENERGY GOES UP
ex. gas expanding/being compressed

If gas expanding doing work on surroundings!
Means work will be positive, it is pushing on what is around it getting bigger which means its energy would go down!
If do the opposite and press on this piston from outside, COMPRESS
Now I am pushing on the thing and using my energy from outside to push on the gas, so the gas’s energy should go up! I am giving energy to the gas by compressing it so here work is being done ON the gas! So work here should be negative, so internal energy would be going up becuase energy from outside force, gas is exerting the force up but here the surroundings are exerting the force down* ENERGY GOES UP

W= P delta V
to find how much work is done on a gas, pressure must be constant!
need to have change in volume, if no change in volume NO work done
sign of change in volume coensides with work done
if Change/delta V> 0= for expanding (POSITIVE WORK)
if change/delta V < 0= for being compressed (NEGATIVE WORK)
work = P x delta V only can do if pressure is constant*
- if delta V is positive, work is positive

what if P isn’t constant for W= P X delta V
JUST FIND area under the curve, which is the work done by a gas
V is getting bigger so delta V is positive so positive work done***
diagram if pressure isnt constant can find work done by gas for area under the curve from a to b** we can find the work done by the gas in that process*

For a cycle if Pressure is not constant
net work doen in a cycle is area inside
KNOW if give you a P delta V diagram work done by the cycle during the cycle** is area inside*

first law of thermodynamics
part 2

heat curve
where to use Q=mCdetaT

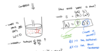
heat engines=
- very complicated*
- start with something hot and something cld; heat will naturally flow river of energy, and what we will do is build a dam, or water wheel around that flow of energy from hot to cold so that we can use some of that flow to do something we want to do like make car move
can burn gas and make stuff hot, heat energy can use to turn into mechanical work to make car go* but importantly some of that heat is lost, can’t fully stop the flow some of that heat will make its way to teh cold place and become exhaust*

actual efficiency
how good engine can turn heat into mechanical work, what we care about! how much work do I actually get from the energy coming out of the hot plate*
- efficieny about hwo built engine, cannot change ratio of those things which is based upon how you made hte engine in the first place like resistance fixed because abotu hwo resistor is made
Qh= heat comes out of hot plate
Qc= heat that goes into cold place, so Qh is the energy that goes into teh engine*** flowing into the engine and Qc is the exhaust* heat
how much work I can do for heat I put in!

carnot efficiency
turns out upper limit to efficiency of any engine, even if in magical physic land no friction, no heat lost everything is perfect all of thsoe completely ridiculous assumptions we make when do physics problems could still not get efficiency of 100% of heat engine
there is some maximum efficiency set by how engine is constructed called carnot efficiency*
idea based on temperature you chose there is some maximum effiency you can have
carnot efficiency 2
maximum efficiency of this engine is: 25% so that is the absolute best that that engine could do the greatest fraction or percent of heat hat goes in can converto to work is 25% if made an engine in real world that had these temperatures it probably would not have an actual efficieny of 25% look on image
teh actualy efficiency is only 5% for every 100 J you put in can only do 5 J of work!

equations

1st law conservation of energy
part 2
- have to put energy in and out otherwise change in energy will be different, change in energy of system must be equal to heat transfer going in and out minus work done by a system* two different ways to change the energy of the system put energy in or out via heat transfer energy/work, want to be careful about with this is becuase the sign conventions

Work
-if system interested and does work, pushing out on surroundings then it is doing positive work* pushing otuward* that means that it is a positive work*
But when I do work I am giving my energy away* my energy goes down* so that is why we need this negative sign, so when the system does work that is a positive work, when thing pushes out* generally in this context we are thinking about expanding when system does work on surroundings that will be by expanding*

ex. gas compressed
if doing work on the system= that will be compressing it* if surroundings do work on system thing is being compressed work is negative getting pushed in on, but when that happens if you are doing work on me my energy should go up so you have minus a negative and change in internal energy is positive*
if gas compressed, work being done on system work is done ON GAS so negative work W<0* internal energy of the gas inc**
expanding doing work! energy goes down*

ex.of gas being compressed 2
if gas compressed, work being done on system work is done ON GAS so negative work W<0* internal energy of the gas inc**
linear expansion
linear expansion- when I heat things up they get bigger, how much bigger depends on property of material/ depends on the material which is represented by alpha coefficient



