Atomic and Nuclear Structure Flashcards
Photoelectric effect 1
light is a particle and a wave, e= Hf
- e- can only absorb [ackets of one at a time
- when think abotu kcicking electrons off a minimum amoutn of energy needed to remove an electron the toll anaology is very helpfuL* either the work function or binding energy or the ionization energy the energy per atom or per electrons that is puer mole** so basically what is happening here idea is that hte photos need to have neough energy to pay the toll
Below the threshold frequency
Not eneough enegy in photon, cannot cross bridge
Doesnt matter how many ppl show up to bridge with 10 dollar bills, if toll is 16 dollars none of hte electrons get to cross and get ejected* cannot add it up together so cannot pool their money can only pull one electron at a time
if not enpugh energy in the photon it will not happen!
idea at low frequencies we have low energy of photos ppl are showing up with not very much money*
At the threshold…..
At the threshold, we are showing up with exactly the right amount of money. we have exaactly what we need to eject the electron no extra!
Above the threshold
conservation of energy! where does the extra money go! so if I show up with 20 dollars and spend 16 on teh toll to get over the bridge, then I have four dollars left over. That 4 dollars needs to go to gas money to making me go faster
it goes to the Ke*
This means because extra energy of each photon goes to KE, as inc frequencies of photons above the threshold we are inc the energies of the photon so therefore there is more extra money left over! 20 dolalrs I have 4, 30 dollars I have 14 more money left over so go faster and faster
inc frequency o flight inc the energy of he photons and inc hte Ke of the ejected electrons** however increasing the intensity dodesnt change how much energy is in each photon**

In terms of intensity…..
just like traffic! should not change how much money ppl show up with, each ppl still show up with the same amount so therefore always the same amount of extra they should all be going the same speed. The only thing that changes then is the number of electrons ejected, if I have more people show up with this amount of energy I will have more people go across the bridge!
Inc intensity the just changes how many people sho wup
inc freqnecies changes energy of each photon, increases the speed! the KE of hte electrons coming off! inc the intensity just changes hte number of photons showing up the number of electrons beingn ejected!!!
1 ev=
1.6 x 10^-19 J
eV is a very small unit of energy

alpha decay
the name of decay is what is emitted
“…” decay means named particle that is emitted

Beta Decay
beta decay, emitting beta particle, emitting an electron
what is happening in beta decay you are converting a neutron to aproton*** start out with 8n and 6 p end with 7n and 7p so taking something that was neutral and splitting it i nto a positive portion adn negative portion leaving behind a proton where you had a neutron*
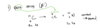
Beta + Decay
positron decay
converting a proton into a neutron
condensing positive to tiny little thing I shoot out and get rid off and I am left with more neutrons

gamma decay
high energy arrangement of protons and neutrons in nuc they rearrange themselves and when they do that they emit a gamma particle*

electron capture
Electron capture, not adecay where electron was absorbed notice this here we have 3N and 4 p this is basically neutralizing a proton to turn it into a neutron electron is absorbed and combinging with a proton to turn it into a neutron* so positive plus negtive becomes neutron** protons in nuc are positive, absorbs negative o felectron and turns it into a neutron*
A CAPTURE
so 0e -1 if says capture means that particle is on the left hand side*

Rate of radioactive decay
NEVER CHANGES EVER
result of factors internal to the nucleus
as a reslt these things have a fixed halflife, amount of time it takes for half of the material to decay for one thing to turn into another thing stays the same, so if I start as I did with 100 milligrams and I wait 1 half life i will have 50 etc….. the point is that the amount of time it takes to lose half of it will stay the same*

E=mc^2
this energy is coming from direct conversion of mass and energy, bits of protons and neutrons are being directly converted into energy! and that energy is what is being given off so we can think abotu how much energy is contained in a certain a mount of mass by using this equation
E=mc^2

4 fundamental forces
- strong nuclear force= attractive force btw protons and neutrons that holds hte nucleus together. inside nucleus strong nuclear force is stronger, but once you leve the nucleus it is very veyr weak only acts at very short distances, so once leave uccleus it doesn’t really do much for you anymore*
- electromagnetic force talking about atraction and forces of positive and negative charges. this is specifcally about attracking force btw positives and negatives
- weak nuclear is responsible for beta decay, so never pick that as an answer unless the question is literally what is responsible for beta decay. Also this should not be a correct answer becuase only applies to things going on inside the nucleus* so if sasking you questions about whats going on inside nucelus! then fine, but unless asking you abotu things specifically inside nucleus this isn’t hte answer and distractor- when I say beta decay he means positron and electron so beta decay plus and minus*
- gravity any two forces with mass attracted, those two forces are really really small so generally ignore them when they are on teh earth but they are there! that is what gravity is!

What is a half life?

ex. of a half life problem using variables x

strong nuclear force and weak nuclear force

half life example 2

Problem set equations

subatomic particles chart

How to balance nuclear equations!

proton
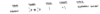
neutron
symbosymbol 1on
mass= 1
charge = 0

electron
mass = 1/1800
charge= -1
notes= aka beta particle, B-

positron
mass= 1/1800
charge= +1
notes= beta + paticle (anitmatter of e-)**

alpha particle
mass = 4
charge= +2
helium nucleus 2P and 2n***

gamma particles
mass= 0
charge = 0
high energy photons

Q16. A 10-W light source emits 1,000 Hz photons. Roughly, ho wmany photons does it emit per minute?

Q16 each photon has specific amount of energy, so need to figure out how many they have.
so because we can think of energy delivered as energy delivered times energy times n of photons. We can replace that in teh formula
SPEFICALLY FOR CASE WHEN POWER SOURCE IS EMITTING PHOTONS** *IF YOU ARE TALKING ABOUT something not emitting photons that wouldnt work* if talking about something giving off energy in discrete packets into photons this will for sure be true*
so the answer is 10^33 REMEMBER THIS EQUATION**

La
Lanthanum
atomic number = 57

Q24. What is the resultant atom if a neutron bombards a gold nucleus and in addition to the daughter nucleus, three alpha particles are emitted?
- so “neutron bombards a gold nucleus” so that means we are starting with gold
- if a neutron is bombarding the gold nucleus means the neutron is in the reactnts, means STRIKES*
oN1 doesnt matter because we are only adding protons, so protons on the other side wil be 79-6= 73 so the atom will have an atomic number, number of protons of 73
the answer is Tantalum

very important equation to use when assked abput how many photons,or protons or electrons….
so if asked “if the mass of a proton is X kg and all of hte mass could be converted into usable energy, how many protons would have to be converted per second to generate 100 W of power?

Q. 25 If the mass of a proton is 1.67 x 10 ^-27 kg and all of the mass could be converted into usable energy, how many protons would have to be converted per second to generate 100W of power?
6.6 x 10^11
remember given a mass so we are using E= mc^2 to get the energy! the energy from matter from converting matter directly into energy!
keep in kg units becuse that is the standard units*

Q. 26 If the mass of an electron is 9.1 x 10^-31 kg and all of that mass could be converted into usable energy, how many electrons would have to be converted per second to generate 1 mW of power?
also here remember making -3 to -4 in scientific notation
KEY= remember making one thing bigger than have to make the exponent smaller***

Q2 from Chem ch 10
When an atom of 235 U iundergos an alpha dcay followed by a beta decay the resut is an atom of:
Pa
so 91 is atomic number becuase it matches the Th90 !!!
U92–> 2He + Th90–> -1 e + Pa 91** equals 90 and that is what you want*

CHem Q8 the decay of C-14 in an archteological sample is best described by which of the following rate laws:
rate= k [14C]
FIRST PORDER****
to lose next 25 % takes just as much as it took to lose first 50% why does that happen, rate going down becuase less of the stuff. so rate laws are always first order for nuclear decays* electron capture would be electrons and thing capturing it so second order*Sn2 ex both things in rate law, sn1 only one thing in rate laaw*
nuclear decay have first order rate laws and depend on the amoutn fo stuff present, true for anythin g that has a constant half life* and the half life remains constant*
Zinc
Zn
atomic number 30
Au
gold
atomic number 79
Ag
Element
atomic number = 47
group 11 period 5
Pb
lead
atomic number 82
Sn
Tin
atomic number 50
Hf
Hafnium
atomic number 72
electron configuration [Xe] 4f¹⁴5d²6s²
Ac
Actinium
atomic number 89
Electron configuration: [Rn] 6d17s2
Ba
Barium
atomic number 56
Rb
Rubidium
atomic number 37
Cs
Caesium
atomic number 55
Ra
Radium
atomic number 88
Cu
Copper
atomic number 29
Co
COBALT NOT COPPER
atomic number 27
REMEMBER A in cobalt comes before PPER**
Cd
Cadium
atomic number 48
Rn
Radon
atomic number 86
RADON IS THE LAST NOBEL GAS**
Kr
krypton
atomic number 36
nobel gas
Sn
Tin
atomic number 50
Tl
Thallium
atomic number 81
post transition metal
Ge
Germanium
atomic number 32
As
arsenic
atomic number 33
Se
Selenium
atomic number 34


