The roles of ATP in living cells and the mechanisms of production of ATP Flashcards
Define metabolism
Metabolism: integrated set of enzymatic reactions comprising both anabolic and catabolic reactions
Define anabolism and catabolism
Anabolism: synthesis of complex molecules from simpler ones (necessary energy usually derived from ATP)
Catabolism: breakdown of energy- rich molecules to simpler ones (CO2 H2O and NH3)
How is energy released from catabolism?
(energy released is ‘captured’ as adenosine triphosphate (ATP) and stored for later use in anabolic reactions
Which processes make up metabolism?
Anabolism: synthetic reactions - the pathways end in ‘genesis’ e.g. glycogenesis (synthesis of glycogen from glucose)
Catabolism: breakdown reactions - the pathways end in ‘lysis’ e.g. glycolysis (breakdown of glucose to pyruvate)
What is energy required for?
§Motion (muscle contraction)
§Transport (of ions/molecules across membranes)
§Biosynthesis of essential metabolites
§Thermoregulation
Why is stored energy needed?
Timing of these energy-requiring processes does not necessarily coincide with feeding times so storage forms of food are required
What is an isothermal system?
- Cells are isothermal systems
- Heat flow cannot be used as a source of energy (heat can only do work when it passes to an area or an object at a lower temperature)
- Free energy (energy available to perform work) is acquired from nutrient molecules
Define
Gibbs free energy
Enthalpy
Entropy
- Gibbs free energy (G) – energy capable of doing work at constant temperature and pressure
- Enthalpy (H) – the heat content of the reacting system
- Entropy (S) – the randomness or disorder in a system
What is the gibbs free energy equation?

What is the gibbs free energy of a reaction?
Gibbs free energy of a reaction – maximum energy that can be obtained from a reaction at constant temperature and pressure
For the reaction A -> B
State what will happen if -
If greater concentration of B than A at equilibrium:
If greater concentration of A than B at equilibrium:

What is an exogernic reaction?
Products have less free energy than reactants and so are more stable than the reactants. Formation of product is “downhill” (spontaneous)
What is an endogernic reaction?
Products have more free energy than reactants and so are less stable than the reactants. Formation of product is ‘uphill’
What kind of reaction is this and is the gibbs free energy positive or negative?


What kind of reaction is this and is the gibbs free energy positive or negative?


What is coupling of reactions?
An endergonic reaction can be driven in the forward direction by coupling it to an exergonic reaction through a common intermediate
How would these reactions be coupled?


What is the role of ATP?
- ATP provides most of the free energy required for anabolism
- ATP is the energy currency of the cell
What is gibbs free energy in relation to ATP?
•Gibbs free energy: the energy derived from the oxidation of dietary fuels to generate ATP
Energy is conserved as _____ and is transduced into useful work
ATP
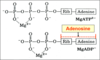
Complete the diagram on the structure of ATP


What is the role of Mg2+ in ATP?
- ATP in the cytosol is present as a complex with Mg2+
- Mg2+ interacts with the oxygens of the triphosphate chain making it susceptible to cleavage in the phosphoryl transfer reactions
What is the result of a Mg2+ deficiency?
A Mg2+ deficiency impairs virtually all metabolism
Label the compounds