TCA Cycle Flashcards
Overall, what is the TCA cycle?
The overall process of glucose metabolism
Glucose is converted to CO2 and water in a reaction that is overall highly exergonic, with many intermediate steps.
Part of the released energy is captured as ATP
Part of the released energy is temporarily stored as NADH
Why does the TCA cycle exist?
Glycolysis can’t be the final step in catabolism
Need to regenerate NAD+ by oxidising NADH in order to metabilise more glucose
Most organisms oxidise pyruvate further, genrally using the TCA cycle
In order to regenerate NAD+, a final electron acceptor is needed to oxidise NADH
- generally, this acceptor is O2
Where does the TCA cycle occur in prokaryotes?
In the cytoplasm
Where does the TCA cycle occur in eularyotes?
In the mitochondrion
What are the four generalised steps of the TCA cycle?
- Oxidation of pyruvate
- The production of isocitrate
- Two decarboxylations
- The regeneration of oxaloacetate
TCA 0:
Reactant
TCA 0:
Reactant: Pyruvate (Pyr)

TCA 0:
Product
TCA 0:
Product: Acetyl-coenzyme A (Ac-S-CoA or Acetyl CoA), NADH + H+, CO2

TCA 0:
Reactant
Product
TCA 0:
Reactant: Pyruvate (Pyr)
Product: Acetyl-coenzyme A (Ac-S-CoA or Acetyl CoA), NADH + H+, CO2

TCA 0:
Reaction type
TCA 0:
Reaction type: Pyruvate oxidation

TCA 0:
Enzyme
TCA 0:
Enzyme: Pyruvate dehydrogenase complex

TCA 0:
Cofactor
TCA 0:
Cofactors: 5 (below)
TPP: decarboxylates pyruvate, yeilds a hydroxyethyl-TPP anion
Lipoic Acid: accepts the hydroxyethyl anion from TPP as an acetyl group (the long arm of lipoamide swings the acetyl group between the active sites of the enzyme complex)
CoA: accepts the acetyl group from acetyl-dihydrolipoamide
FAD: reduced by dihydrolipoamide
NAD+ : reduced by FADH2

TCA 0:
Reactant
Product
Reaction type
Enzyme
Cofactor
TCA 0:
Reactant: Pyruvate (Pyr)
Product: Acetyl-coenzyme A (Ac-S-CoA or Acetyl CoA), NADH + H+, CO2
Reaction type: Pyruvate oxidation
Enzyme: Pyruvate dehydrogenase complex
Cofactors: 5 (below)
TPP: decarboxylates pyruvate, yeilds a hydroxyethyl-TPP anion
Lipoic Acid: accepts the hydroxyethyl anion from TPP as an acetyl group (the long arm of lipoamide swings the acetyl group between the active sites of the enzyme complex)
CoA: accepts the acetyl group from acetyl-dihydrolipoamide
FAD: reduced by dihydrolipoamide
NAD+ : reduced by FADH2

What is glucose converted to in the TCA cycle?
Glucose is oxidized as far as it can go, to CO2 and H2O
TPP
Thiamine pyrophosphate (TPP) a thiamine (vitamin B1) derivative which is a cofactor that is present in all living systems, in which it catalyzes several biochemical reactions. It is an essential nutrient (vitamin) in humans.
TPP works as a coenzyme in many enzymatic reactions, such as:
Pyruvate dehydrogenase complex:
- decarboxylates pyruvate, yeilds a hydroxyethyl-TPP anion
Pyruvate decarboxylase in ethanol fermentation
Alpha-ketoglutarate dehydrogenase complex
Branched-chain amino acid dehydrogenase complex
2-hydroxyphytanoyl-CoA lyase
Transketolase

Lipoic Acid
Lipoic acid or lipoate
The lipoyllysyl moiety is the prosthetic group of dihydrolipoyl transacetylase (E2 of the PDH complex). The lipoyl group occurs in oxidised (disulfide) and reduced (dithiol) forms and acts as a carrier of both hydrogen and an acetyl (or other acyl) group.

CoA
Coenzyme A (CoA, CoASH, or HSCoA) is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle.
In the pyruvate dehydrogenase complex, accepts the acetyl group from acetyl-dihydrolipoamide

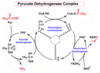
PDC
The pyruvate dehydrogenase complex (PDC) serves as the enzymatic gatekeeper facilitating and regulating entry into the citric acid cycle for metabolites leaving glycolysis.
PDC is composed of multiple copies of three enzymes: pyruvate dehydrogenase (E1) (with its bound cofactor TPP); dihydrolipoyl transacetylase (E2) (with its covalently bound lipoyl group); and dihydrolipoyl dehydrogenase (E3) (with its cofactors FAD and NAD).
PDC E1
pyruvate dehydrogenase, E1 (with its bound cofactor TPP)
E1 catalyzes first the decarboxylation of pyruvate, producing hydroxyethyl-TPP, and then the oxidation of the hydroxyethyl group to an acetyl group. The electrons from this oxidation reduce the disulfide of lipoate bound to E2, and the acetyl group is transferred into thioester linkage with one — SH group of reduced lipoate.

PDC E2
dihydrolipoyl transacetylase, E2 (with its covalently bound cofactor lipoate)
E2 catalyzes the transfer of the acetyl group to coenzyme A, forming acetyl-CoA.
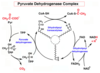
PDC E3
dihydrolipoyl dehydrogenase, E3 (with its cofactors FAD and NAD+)
E3 catalyzes the regeneration of the disulfide (oxidized) form of lipoate; electrons pass first to FAD, then to NAD+, forming NADH + H+

dihydrolipoyl dehydrogenase
dihydrolipoyl dehydrogenase, E3 (with its cofactors FAD and NAD+)
E3 catalyzes the regeneration of the disulfide (oxidized) form of lipoate; electrons pass first to FAD, then to NAD+, forming NADH + H+
pyruvate dehydrogenase
pyruvate dehydrogenase, E1 (with its bound cofactor TPP)
E1 catalyzes first the decarboxylation of pyruvate, producing hydroxyethyl-TPP, and then the oxidation of the hydroxyethyl group to an acetyl group. The electrons from this oxidation reduce the disulfide of lipoate bound to E2, and the acetyl group is transferred into thioester linkage with one — SH group of reduced lipoate.
dihydrolipoyl transacetylase
dihydrolipoyl transacetylase, E2 (with its covalently bound cofactor lipoate)
E2 catalyzes the transfer of the acetyl group to coenzyme A, forming acetyl-CoA.

How are intermediates shuffled through the pyruvate dehydrogenase complex?
The long lipoyllysyl (lipoic acid + lysine) arm swings from the active site of E1 to E2 to E3, tethering the intermediates to the enzyme complex to allow substrate channeling.

What is the entry point to the TCA cycle?
Acetyl-Coenzyme A
High-energy thioester bond:
DG°’ = -32.2 kJ/mol
Four electron pairs (in blue) of acetyl-CoA that are ultimately used to reduce NAD+ (3) and FAD (1) in the Citric Acid Cycle

TCA I
Reactant
TCA I
Reactant: Acteyl-Coenzyme A (AcCoA) + Oxaloacetate (OxAc)

TCA I
Product
TCA I
Product: Citrate (Cit)

TCA I
Reactant
Product
TCA I
Reactant: Acteyl-Coenzyme A (AcCoA) + Oxaloacetate (OxAc)
Product: Citrate (Cit)

TCA I
Type of Reaction
Enzyme
Cofactor
TCA I
Type of Reaction: Condensation
Enzyme: Citrate synthase
Cofactor: none (remember water is required because this is a condensation reaction)

TCA I
Enzyme
TCA I
Enzyme: Citrate synthase
(synthases: enzymes that catalyze condensation reactions but do not require ATP)
•This enzyme is a dimer that binds oxaloacetate first, then acetyl-CoA. Hence, an ordered bisubstrate reaction mechanism or “induced fit.”

TCA I
Cofactor
TCA I
Cofactor: none (remember water is required because this is a condensation reaction)

TCA I
Reactant
Product
Type of Reaction
Enzyme
Cofactor
TCA I
Reactant: Acteyl-Coenzyme A (AcCoA) + Oxaloacetate (OxAc)
Product: Citrate (Cit)
Type of Reaction: Condensation
Enzyme: Citrate synthase
Cofactor: none (remember water is required because this is a condensation reaction)

citrate synthase reaction mechanism
Citrate synthase is a dimer that binds oxaloacetate first, then acetyl-CoA. Hence, an ordered bisubstrate reaction mechanism or “induced fit.”
Found in TCA I


Citrate (Cit)

Isocitrate
Structure of citrate

Structure of isocitrate

Structure of pyruvate

Where is pyruvate decarboxylated?
While bound to thiamine pyrophosphate (TPP) on the E1 complex of pyruvate dehydrogenase complex.

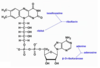
Subunits of Coenzyme A
3’-AMP
pantothenic acid
β-mercaptoethylamine

Acetyl Coenzyme A structure

FAD name and structure
Flavin Adenine Dinucleotide
Where is FAD reduced?

What structural modification does pyruvate dehydrogenase complex perform?
Removes CO2 from pyruvate, generates NADH + H+

TCA II
Reactant
TCA II
Reactant: Citrate

TCA II
Product
TCA II
Product: Isocitrate

TCA II
Reactant
Product
TCA II
Reactant: Citrate
Product: Isocitrate

TCA II
Type of Reaction
Enzyme
Cofactor
TCA II
Type of Reaction: Isomerisation

TCA II
Enzyme
TCA II
Enzyme: Aconitase

TCA II
Cofactor
TCA II
Cofactor: none (acontiase dehydrates to form a double bond then hydrates the same double bond on the opposite carbon to isomerise the molecule)

TCA II
Reactant
Product
Type of Reaction
Enzyme
Cofactor
TCA II
Reactant: Citrate
Product: Isocitrate
Type of Reaction: Isomerisation
Enzyme: Aconitase
Cofactor: none (acontiase dehydrates to form a double bond then hydrates the same double bond on the opposite carbon to isomerise the molecule)

Why is citrate prochiral?
Because of the hydroxyl (-OH) group, only one CH2COO- is susceptable to attack due to the conformation of the active site. There is only one way in which the three specified groups of citrate can fit on the three points of the binding site.

TCA III
Reactant
TCA III
Reactant: Isocitrate

TCA III
Product
TCA III
Product: α-Ketoglutarate, NADH + H+, CO2

TCA III
Reactant
Product
TCA III
Reactant: Isocitrate
Product: α-Ketoglutarate, NADH + H+, CO2

TCA III
Type of Reaction
TCA III
Type of Reaction: Decarboxylation

TCA III
Enzyme
TCA III
Enzyme: Isocitrate dehydrogenase

TCA III
Cofactor
TCA III
Cofactors: NAD+, then H+

TCA III
Reactant
Product
Type of Reaction
Enzyme
Cofactor
TCA III
Reactant: Isocitrate
Product: α-Ketoglutarate, NADH + H+, CO2
Type of Reaction: β-cleavage of carboxy group
Enzyme: Isocitrate dehydrogenase
Cofactors: NAD+, then H+

TCA IV
Reactant
TCA IV:
Reactant: α-Ketogluterate

TCA IV
Product
TCA IV:
Product: Succinyl-coenzyme A (Suc-CoA), NADH + H+, CO2

TCA IV
Reactant
Product
TCA IV:
Reactant: α-Ketogluterate
Product: Succinyl-coenzyme A (Suc-CoA), NADH + H+, CO2

TCA IV
Type of Reaction
TCA IV:
Type of Reaction: β-cleavage of carboxy group

TCA IV
Enzyme
TCA IV:
Enzyme: α-Ketogluterate dehydrogenase complex

TCA IV
Cofactor
TCA IV:

Cofactors: TPP, Lipoic Acid, CoA, FAD, NAD+
TCA IV
Reactant
Product
Type of Reaction
Enzyme
Cofactor
TCA IV:
Reactant: α-Ketogluterate
Product: Succinyl-coenzyme A (Suc-CoA), NADH + H+, CO2
Reaction type: Decarboxylation
Enzyme: α-Ketogluterate dehydrogenase complex
Cofactors: TPP, Lipoic Acid, CoA, FAD, NAD+
∆G°’ = -33.4 kJ/mol

Succinyl-CoA structure


Succinyl-CoA
TCA V
Reactant
TCA V
Reactant: Succinyl-CoA

TCA V
Product
TCA V
Product: Succinate + GTP (in mammals, else ATP) + CoA-SH

TCA V
Reactant
Product
TCA V
Reactant: Succinyl-CoA
Product: Succinate + GTP (in mammals, else ATP) + CoA-SH

TCA V
Type of Reaction
TCA V
Type of Reaction: Hydrolysis

TCA V
Enzyme
Cofactor
TCA V
Enzyme: Succinyl CoA synthetase
Cofactor: GDP + Pi (in mammals, else ATP + Pi)
∆G°’ = -2.9kJ/mol

TCA V
Cofactor
TCA V
Cofactor: GDP + Pi (in mammals, else ATP + Pi)

TCA V
Reactant
Product
Type of Reaction
Enzyme
Cofactor
TCA V
Reactant: Succinyl-CoA
Product: Succinate + GTP (in mammals, else ATP) + CoA-SH
Type of Reaction: Hydrolysis
Enzyme: Succinyl CoA synthetase
Cofactor: GDP + Pi (in mammals, else ATP + Pi)
∆G°’ = -2.9kJ/mol

TCA VI
Reactant
TCA VI
Reactant: Succinate (Suc)

TCA VI
Product
TCA VI
Product: Fumarate (Fum) + FADH2

TCA VI
Type of Reaction
TCA VI
Type of Reaction: Dehydrogenation

TCA VI
Enzyme
TCA VI
Enzyme: Succinate dehydrogenase

TCA VI
Reactant
Product
TCA VI
Reactant: Succinate (Suc)
Product: Fumarate (Fum) + FADH2

TCA VI
Cofactor
TCA VI
Cofactor: FAD

TCA VI
Reactant
Product
Type of Reaction
Enzyme
Cofactor
TCA VI
Reactant: Succinate (Suc)
Product: Fumarate (Fum) + FADH2
Type of Reaction: Dehydrogenation
Enzyme: Succinate dehydrogenase
Cofactor: FAD
∆G°’ = 0 kJ/mol

TCA VII
Reactant
TCA VII
Reactant: Fumarate

TCA VII
Product
TCA VII
Product: L-Malate (Mal)

TCA VII
Reactant
Product
TCA VII
Reactant: Fumarate
Product: L-Malate (Mal)

TCA VII
Type of Reaction
TCA VII
Type of Reaction: Hydration of alkene

TCA VII
Enzyme
TCA VII
Enzyme: Fumarase

TCA VII
Cofactor
TCA VII
Cofactor: none (water required for hydration)

TCA VII
Reactant
Product
Type of Reaction
Enzyme
Cofactor
TCA VII
Reactant: Fumarate
Product: L-Malate (Mal)
Type of Reaction: Hydration of alkene
Enzyme: Fumarase
Cofactor: none (water required for hydration)
∆G°’ = -3.8 kJ/mol

TCA VIII
Reactant
TCA VIII
Reactant: L-Malate

TCA VIII
Product
TCA VIII
Reactant: L-Malate
Product: Oxaloacetate, NADH + H+
Type of Reaction: Dehydrogenation
Enzyme: L-Malate dehydrogenase
Cofactor: NAD+
∆G°’ = +29.7 kJ/mol

TCA VIII
Reactant
Product
TCA VIII
Reactant: L-Malate
Product: Oxaloacetate, NADH + H+

TCA VIII
Type of Reaction
TCA VIII
Type of Reaction: Dehydrogenation

TCA VIII
Enzyme
TCA VIII
Enzyme: L-Malate dehydrogenase

TCA VIII
Cofactor
TCA VIII
Cofactor: NAD+

TCA VIII
Reactant
Product
Type of Reaction
Enzyme
Cofactor
TCA VIII
Reactant: L-Malate
Product: Oxaloacetate, NADH + H+
Type of Reaction: Dehydrogenation
Enzyme: L-Malate dehydrogenase
Cofactor: NAD+
∆G°’ = +29.7 kJ/mol

Summize the TCA cycle
- Acetyl CoA from pyruvate condenses with oxaloacetate, a 4-C dicarboxylic acid to form citrate, a 6-C tricarboxylic acid
- The citrate rearranges to form isocitrate
- Isocitrate is oxidatively decarboxylated, yielding CO2 α-ketoglutarate (a 5-C dicarboxylic acid), and NADH
- α-ketoglutarate is oxidatively decarboxylated to yield CO2, succinyl CoA (a derivative of a 4 carbon dicarboxylic acid) and NADH
- Succinyl CoA is hydrolyzed to succinate and CoA, yielding one molecule of ATP
- Succinate is converted in three steps to oxaloacetate
–The succinate is oxidized to yield fumarate, a 4-C dicarboxylic acid and FADH2.
–Water is added to fumarate to make malate
–Malate is oxidized to yield oxaloacetate and NADH.
Describe β cleavage
Abstract proton from β-hydroxyl group, collapsing resulting C-O bond to a carbonyl, which leaves. Resonance stabilised carbanion is reprotonated to form methyl group.

How many ATP per NADH reduced are formed from glycolysis to PDC through TCA and Oxidative phosphorylation? Per FADH2 reduced?
2.5 ATP/NADH and 1.5 ATP/FADH2
about 34% (32 x 30.5 kJ/mol = 976 kJ/mol) of the available energy (2,840 kJ/mol) in glucose is recovered
What are the rate-limiting enzymes of the TCA cycle?
Citrate Synthase, Isocitrate Dehydrogenase and α-ketoglutarate Dehydrogenase
Unlike the rate limiting enzymes of glycolysis, which use elaborate systems of allosteric control and covalent modification as flux control mechanisms, the citric cycle rate-limiting enzymes are largely regulated by:
1) substrate availability
2) product inhibition
3) inhibition by other cycle intermediates.
Major regulators are: its substrates (acetyl CoA, oxaloacetate), its product (NADH).
What do the rate-limiting enzymes of the TCA cycle have in common?
They all reduce NADH, and are the only ones in the TCA cycle that do

“product inhibition”
What inhibits the TCA cycle?
High ATP/NADH
(High energy levels)
What activates the TCA cycle?
Low ATP or high AMP
(Low energy levels)

α-Ketoglutarate
α-Ketoglutarate structure


Oxyacetate (OxAc)
Oxyacetate structure
(OxAc)


Fumarate (Fum)
Fumarate structure
(Fum)



