SPR L4 Analagesia and Gut Motility Drugs Flashcards
Learning Outcomes
- Describe the ways in which different analgesics target the neural pain mechanisms
- Apply the analgesic ladder in hypothetical clinical scenarios of uncontrolled pain
- Contrast the different uses and adverse effects of common simple analgesics and opioid drugs
- Describe the different targets for anti-emetic drugs on the emesis signals
- Discuss different modes on purgative / laxative
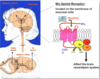
Pain assessment
Outline the difficulties with assessing pain, and how it should be carried out
Pain can be very hard to describe, clinicians often underestimate pain, Must hear what the patient is saying and how he/she is saying it
- Characterize the pains, Identify their mechanisms and causes
- Assess their impact on the patient’s functioning and QOL
- Use a PAIN SCALE (see picture)
NB: When pain does not respond to usual therapy, re-evaluate the patient for causes that may have been missed.

What are the different types of pain?
- Nociceptive pain
- tissue damage (two types)
- Somatic e.g. metastatic bone pain
- Visceral e.g. liver capsule pain, e.g. colic from malignant bowel obstruction
- tissue damage (two types)
- Neuropathic
- nerve damage
- Peripheral or central
- Dysaesthetic (burning)
- Lancinating
Evaluate the use of pain scores
Some of these scales are embedded in tools to assess pain and other sympotms (e.g. Edmonton Symptom Assessment Scale-ESAS, Brief Pain Inventory-BPI, etc). Adopt one and use it routinely. Scales should be used routinely and documented in patient charts.
Having patients keep a pain diary – recording time, level of pain, activity, medication taken, relief obtained – can be very useful.
When analyzing these scales, consider the multidimensionality of the pain experience.
While they are subjective, they generally provide a consistent measurement for that individual patient over time.
Cancer Pain Management: WHO Pain Ladder
Outline the steps in this pain ladder
- First ask participants if they are acquainted with the WHO ladder and ask them to briefly describe its applications.
- Step 1 = “mild” pain (1-3/10 and not affecting functioning or quality of life significantly)
- Step 2 = “moderate” pain (4-6/10 with moderate impact on functioning and quality of life)
- Step 3 is for “severe” pain. Note that one may “go directly” to Step 2 or even Step 3 if pain is severe.
Ask the learners to give examples of drugs for each step.
Step 1: Aspirin, NSAI, acetaminophen
Step 2: Codeine, Percocet & Percodan (i.e combinations of oxycodone with aspirin or acetaminophen). While oxycodone is a strong opioid and is 1 and ½ to 2 times more potent than morphine, the combinations with aspiriin or acetaminophen does not allow one to increase the dose of oxycodone to the degree one could if it was oxycodone alone.
Step 3: Morphine, oxycodone, hydromorphone, fentanyl, methadone. Note, do not use meperidine.
Some have suggested the addition of a fourth ladder for those with intractable pain. Treatments at this level should include intraspinal treatments, special nerve blocks such as celiac plexus blocks for pancreatic and gastric cancer-related pain and cordotomies.

Give examples of
- Simple Analgesics
- Opioid Agonists
- Strong Opioid Agonists
These manage mild (1) to severe pain (3)
- Paracetamol (NSAIDs)
- Codeine, Dihydrocodeine and Tramadol
- Morphine and Diamorphine
- What is the classification of Paracetamol?
- What is it’s mode of action?
- analgesic, antipyretic, misc. not an NSAID
- inhibits prostaglandin synthesis via CNS inhibition of COX3 (not peripheral)— doesn’t promote ulcers, bleeding or renal failure; peripherally blocks generation of pain impulses, inhibits hypothalamic heat-regulation center
(paracetamol is NOT an example of an NSAID - doesnt have an anti-inflammatory effect)
PARACETAMOL
- What is it?
- What is it’s mode of action?
- What are the indications for it’s use?
- What are the adverse effects?
- Active metabolite of phenacetin
- Analgesic - Weak prostaglandin inhibitor
- Mild to moderate pain without inflammation
- Rare at normal dosage
Caution in liver impairment/low body weight
Liver/renal toxicity in over dosage
Paracetamol
Outline how it is broken down and how it can cause damage
Underweight = less glutathione

OPIATE ANALGESICS
- What is the source?
- What are the principal alkaloids?
- What is their classification?
- Seed pods of the opium poppy
- Morphine and Codeine
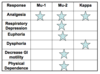
- Full agonists - High efficacy/strong agonists
Partial agonists – Weaker analgesia /antagonist
Prescribing Opioids
- Weak opioids - what are the general principles?
- Strong Opioids - give examples
- What SHOULD NOT be used?
- Dose often, Codeine limited by presence of paracetamol and constipation, Tramadol (caution in elderly and with SSRIs).
- Morphine, oxycodone, diamorphine, fentanyl, hydromorphone, methadone, buprenorphine
- Do NOT use - Pethidine
- What dosing is required for short-acting (immediate release opioids)
- What dosing is required for long acting opioids formulations of morhpine, oxycodone, hydromorphone and codeine?
- When is steady state usually achieved?
- 4 hourly dosing.
- 12 hourly dosing.
- within 5 times the half life. Since the half lives (the time it takes for the levels in the blood to reduce by half) are generally around 4.5 to 6 hours, it will take about 24hrs to achieve steady state with controlled release formulations of morphine. In the case of fentanyl transdermally, the half life is approximately 12hr to 17 hrs. Steady state is therefore only achieved after up to 80 hours. For this reason, doses of patches should not be changed more frequently than every 72 hours.
(There are some exceptions to the 12 hourly dosing of sustained release formulations of codeine, morphine, hydromorphone and oxycodone and the 72hourly dosing of transdermal fentanyl. Approximately 10% to 15% will demonstrate end of dose failure despite increases in dosing. In these select cases, the dosing frequency may need to be increased to every 8hrs and 48 hrs respectively. The frequency of the dose is only increased when end of dose failure, characterized by increased pain the last few hours prior to the next dose, manifests despite at least 3 attempts at increasing the dose (amount). )
Outline the differences between opioids
There exists wide inter- and intra-individual variability in responses (both analgesic and adverse effects) to various opioids.
If 10 patients of the same age and exactly the same disease process, experiencing the same amount of pain, were given each a regimen of morphine 5mg every 4 hours and then titrated, one would see a lot of variation in their responses to the opioid. Some may experience excellent pain control with no side effects, others may experience mild side effects, while others may experience no benefits and more significant side effects. One patient may do better on morphine than on hydromorphone, while the opposite may be true for another.
Prescribing opioids - general principles (for general persual)
Neurotoxicity in patients with renal impairment requiring opioid treatment often improves when patients are switched from morphine to hydromorphone or fentanyl. The improvement is however probably more related to the switch or opioid rotation rather than inherent properties of hydromorphone or fentanyl. Whether or not hydromorphone, fentanyl and methadone do indeed cause less neurotoxicity than morphine still needs to be assessed in larger studies designed to specifically address the question.
Some very small open studies have suggested that oxycodone and hydromorphone may cause less confusion than morphine. This needs to be confirmed in larger studies.
Studies have not shown fentanyl to be less nauseating than the other opioids. However, chronic opioid-induced nausea may be relieved when a patient’s opioid is switched. The improvement is not from inherent properties of the new opioid, but rather from the switch itself.
In theory methadone may have advantages over other opioids for manaing neuropathic pain (NMDA antagonism) but this is still to be confirmed in studies.
There are no good studies to show that oxycodone has a special role for bone or arthritis-related pain.
All opioids are constipating, which one is less constipating?
fentanyl
What do the following mean?
- opium
- opiate
- opioid
- a Greek word meaning “juice,” or the exudate from the poppy
- a drug extracted from the exudate of the poppy
- a natural or synthetic drug that binds to opioid receptors producing agonist effects
Where do natural opioids occur?
What is the source of other opioid?
- In the juice of the opium poppy (morphine and codeine)
- As endogenous endorphins
All other opioids are prepared from either morphine (semisynthetic opioids such as heroin) or they are synthesized from precursor compounds (synthetic opioids such as fentanyl)
Outline the pharmacological effects of Opiods?
-
Sedation and anxiolytic
- Drowsiness and lethargy
- Apathy
- Cognitive impairment
- Sense of tranquility
-
Depression of respiration
- Main cause of death from opioid overdose
- Combination of opioids and alcohol is especially dangerous
-
Cough suppression
- Opioids suppress the “cough center” in the brain
-
Pupillary constriction
- pupillary constriction in the presence of analgesics is characteristic of opioid use
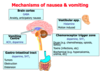
- Nausea and vomiting
- Stimulation of receptors in an area of the medulla called the chemoreceptor trigger zone causes nausea and vomiting
- Unpleasant side effect, but not life threatening
- Gastrointestinal symptoms
- Opioids relieve diarrhoea as a result of their direct actions on the intestines
- Other effects
- Opioids can release histamines causing itching or more severe allergic reactions including bronchoconstriction
- Opioids can affect white blood cell function and immune function
What is the mechanism of action of Opioids?
Activation of peripheral nociceptive fibers causes release of substance P and other pain-signaling neurotransmitters from nerve terminals in the dorsal horn of the spinal cord
- Release of pain-signaling neurotransmitters is regulated by endogenous endorphins or by exogenous opioid agonists by acting presynaptically to inhibit substance P release, causing analgesia

Mode of Action of Morphine
- What is the target?
- What is it’s action?
- What is it’s effect?
- What is the overall effect achieved?
- G-protein coupled opioid ‘mu’ receptors in the CNS and PNS
- Full agonist (high affinity for receptors)
- Closure of N-type voltage dependent calcium channels and opening of calcium dependent inwardly rectifying K+ channels- this increases K conductance. Opioids also presynaptically inhibit the release of pain signalling neurotransmitters such as substance P
- Membrane hyperpolarisation and reduced neuronal excitability, reduction or inhibition of pain neurotransmission