SPR L12 ADRs and Drug Interactions Flashcards
Learning Outcomes
for general perusal
- Describe the different ways in which drugs can interact with each other
- List the factors that increase the risk of drug interactions
- Describe the common adverse drug effects and how to manage them
- Discuss how you would go about reporting a suspected adverse drug effect in the UK
Outline of the lecture
- Highlight common culprits
- Discuss Type A and Type B adverse drug reactions (ADRs) and how they differ
- Elements that can influence the probability of an ADR occurring
- How to manage an ADR
- Look at common drug interactions and how to minimise these
Adverse drug reactions: Epidemiology
- ADRs account for what percentage of hospital admissions and inpatients?
- Cause of death in what percentage of patients?
- What harm do they cause?
- Account for 5% of hospital admissions, occur in 10% of hospital inpatients
- 0.1% of medical and 0.01% of surgical patients
- Adversely affect quality of life, May mimic disease leading to unnecessary investigations or treatment, Cause patients to lose confidence
Intensive hospital monitoring of adverse reactions to drugs
Monitoring patients in the medical, surgical and dermatology wards of the Belfast City Hospital from admission to discharge.. A total of 506 patients were surveyed February to August 2001.
- What percentage of patients experienced ADRs in hospital?
- What were the commonest calsses of drugs causing ADRs?
- What were the drugs most commonly involved with severe ADRs?
- 16.6% of patients experienced ADRs in hospital (most were mild/moderate only).
- antibiotics, analgesics and anticoagulants.
- hypoglycaemic agents (insulin) and NSAIDS.
Nature of ADRs
Outline the nature of ADRS
- Nausea and vomiting
- Excessive sedation
- Rash
- Bleeding
- Renal failure / electrolyte abnormalities
- Abnormal liver enzymes
Drugs commonly causing ADRs
Give examples of the following drugs that especially cause ADRS
- Antibiotics
- Anticoagulants
- Analgesics
- Antihypertensives
nAntibiotics especially:
nFlucloxacillin / Co-amoxiclav
nClarithromycin
nCeftazidime
- Warfarin / LMWHeparin
- Morphine / Tramadol / Co-codamol 30/500, Ibuprofen other NSAIDs
- ACE inhibitors / ARBs, Diuretics Alpha blockers
Classificiation of ADRs
Type A (Augmented / accentuated)
Type B (bizarre)
Type C (Chronic)
Type D (Delayed)
Type E (End-of-Use)
Type F (Failure)
Classification of ADR
What do the following mean?
- Type A
- Type B
- Type C
- Type D
- Type E
- Type F
- Augmented / accentuated
- bizarre
- Chronic
- Delayed
- End-of-Use
- Failure
Type A - Augmented
Give the main characteristics of these reactions
- Dose-dependent
- Predictable from pharmacology of the drug
- Host independent
- Common
- Usually mild
- Low morbidity and mortality
- Reproducible in animal studies
- E.g. Bleeding/bruising on warfarin or aspirin
Reduce dose or withhold
Type A - Augmented
Mechanisms of Type A ADRs
- Outline the pharmacokinetic mechanism
- Outline the pharacodynamic mechanisms
- Pharamceutical mechanisms?
- renal excretion, hepatic metabolism, extremes of age, genetic variations
- genetic variations, extremes of age
- excipients (an inactive substance that serves as the vehicle or medium for a drug or other active substance), bioequivalence (is a term in pharmacokinetics used to assess the expected in vivo biological equivalence of two proprietary preparations of a drug. If two products are said to be bioequivalent it means that they would be expected to be, for all intents and purposes, the same.)

Cytochrome P450
Give the substrates (1) and inhibitors (2) for each of these enzymes
- CYP1A2
- CYP2D6
- CYP3A4
- CYP1A2
- Clozapine, R-warfarin, Ciprofloxacin, Theophylline
- Cimetidine, Erythromycin
- CYP2D6
- Amitriptyline Phenytoin S-warfarin
- Amiodarone Cimetidine Fluconazole
- CYP3A4
- Carbamazepine Cyclosporin Erythromycin
Lignocaine Terfenidine Verapamil
2. Cimetidine Clarithromycin Erythromycin Grapefruit juice Ketoconazole

Type A - Augmented
Outline this
- Poor metabolizers
- Extensive metabolisers
- CYP2D6
- CYP2C19
- acetylation “fast”/”slow”
- methylation
- non-hepatic metabolism (pseudocholinesterase)
Type B (Bizzarre)
- Give an examples
- Give an overview of the characterisitics of this type of ADR
- What can be done?
- E.g. Anaphylaxis to penicillin
2.
- Dose independent
- Unpredictable
- Host dependent
- Uncommon
- Can be severe
- High morbidity and mortality
- No animal models
- E.g. Anaphylaxis to penicillin
- withhold and avoid in future / put in notes
Allergic reactions
(Hypersensitivity reactions)
What are the following types of hypersensitivity reactions?
- Type I
- Type II
- Type III
- Type IV
- immediate: IgE => mast cell release => anaphylaxis
- antibody-mediated cytotoxic: drug induced haemolysis
- immune complex: fever, rash, arthropathy, glomerular damage
- delayed/cell mediated: drug acts as hapten, rash common
(Haptens are small molecules that elicit an immune response only when attached to a large carrier such as a protein; the carrier may be one that also does not elicit an immune response by itself. (In general, only large molecules, infectious agents, or insoluble foreign matter can elicit an immune response in the body.)
A typical drug induced rash

This rash is one of the most common drug-induced skin reactions seen in clinical practice. Patients may be asymptomatic or report itching, burning, or pain. The most common cutaneous manifestation is palpable purpura that are typically round and 1-3 mm. They may coalesce to form plaques (shown) or ulcerate and are most commonly found on the legs. The drugs most commonly responsible are antibiotics, particularly beta-lactams; NSAIDs; and diuretics. Leukocytoclastic vasculitis, also known as hypersensitivity vasculitis or hypersensitivity angiitis, is a small-vessel vasculitis that manifests in the skin, joints, gastrointestinal tract, or kidneys. Removal of the offending drug will typically cause lesions to disappear in up to 2 weeks. Elevation of dependent areas or the use of compressive stockings may be helpful.
Rare but Severe skin reaction:
Stevens-Johnson syndrome
What is this?
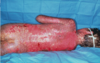
Drug reactions can mimic a wide range of dermatoses with morphologies that include morbilliform, urticarial, papulosquamous, pustular, and bullous lesions. The overall incidence of adverse cutaneous reactions to drugs is estimated to be 0.1%-2.2%. However, semisynthetic penicillins and trimethoprim/sulfamethoxazole may have an incidence as high as 3%-5%. Patients with HIV may also be at increased risk. For all patients with a suspected drug reaction, a detailed history of all medications, including over-the-counter and herbal remedies, taken over the last several months must be obtained. The diagnosis for cutaneous drug reactions is typically made based on a careful history and the appearance of the skin findings. The image shown is of an individual with Stevens-Johnson syndrome, a severe drug-induced bullous reaction that can lead to significant morbidity or mortality.
Photosensitivity reaction
What are the three mechanisms?

The pathogenesis underlying drug-related dyspigmentation can also be categorized into 3 mechanisms, which are
- drug or drug metabolite deposition in the dermis and epidermis
- enhanced melanin production with or without an increase in the number of active melanocytes
- drug-induced postinflammatory changes to skin.
Antimalarials, chemotherapeutic agents, heavy metals, miscellaneous medications (eg, amiodarone, zidovudine, minocycline, clofazimine, psoralens), and psychotropic drugs are among the most commonly implicated medications in acquired dyschromia.
Drug-induced photosensitivity
What are the most common causes?
- Antimalarials
- chemotherapeutic agents
- psychotropic drugs
- heavy metals
- miscellaneous medications (eg, amiodarone, zidovudine, minocycline, psoralens)
Pseudoallergic reactions
- What are these?
- If severe, what may they be termed?
- When is this type of reaction common?
- Mimic allergic reactions, especially Type I, similar clinical features but no evidence of immune response.
- if severe may be termed anaphylactoid
- when Miscalculating N-Acetyl cysteine regimen
- Always double check your calculations with someone-else
Pharmacogenetics in Type B ADRs
Give an onverview
- Glucose-6-phosphate dehydrogenase deficiency
- haemolysis of RBCs with oxidizing agents
- Porphyria
- Malignant hyperthermia
- 1:20 000, abnormal response to GA
- Coumarin (warfarin) resistance
- Aminoglycoside induced deafness
- Long QT Syndrome
Type C (Chronic / cumulative)
- Describe this reaction
- Give an example
- What can be done?
- Uncommon, Related to cumulative dose
- Adrenal suppression on long term corticosteroids
- Withdrawal may need to carried out slowly
Type D (Delayed)
- Describe this reaction
- When does this occur?
- What is it often related to?
- Give an example
- What is it often?
- Uncommon
- Occurs some time after the drug has been started
- Often dose and time related
- E.g. Carcinogenesis or Tardive dyskinesia
- Often intractable
Type E (End-of-use)
- Describe this
- Give an example
- What needs to be done?
- Uncommon, Withdrawal effect, Occurs shortly after drug is stopped
- E.g. Opiate withdrawal syndrome, MI off betablocker
- Re-introduce drug and wean down slowly
Type F (failure)
- What is this usually related to?
- Give an example
- What needs to be done?
- usually dose related - May be due to drug interactions
- Failure of OCP due to enzyme induction
- Increase dose and consider potential drug interactions
Identifying Adverse Drug Reactions
What can be used to identify ADRs?
- Timing of administration
- Previous evidence in literature
- Absence of alternative explanation
- Effects of re-challange

Reporting ADRs
- How can these be reported?
- What will this involve?
- What should be done with new drugs?

- Report to Committee on Safety of Medicines (MHRA) by Yellow Card scheme
- Established drugs report “all serious suspected reactions”, i.e., fatal, life-threatening, disabling, or which prolong hospital stay “even if the effect is well recognised.”
- report all suspected reactions, i.e., any adverse or unexpected event however minor which could conceivably be attributed to the drug.
Management of a suspected ADR
How should an ADR be managed?
- Identify problem as an ADR
- Remove offending drug(s)
- Provide supportive treatment as required
- Check no error has occurred
- Report ADR to MHRA (yellow card) if appropriate
- Write in Kardex / medical notes & inform GP
ADRs: summary
- A common cause of morbidity and mortality.
- Can be predictable from known pharmacological effect (Type A) or may be idiosyncratic / unpredictable (Type B) less commonly other type C-F
- Careful prescribing with sound knowledge of drug and knowledge of patient may prevent ADRs.
- Remember to report suspected ADRs.
Drug Interactions
- What percentage of ADR do these account for?
- Give characterisitics
- What are the mechanisms of drug interactions?
- 15%
- Common, Avoidable
- Pharmaceutical
Pharmacokinetic
Pharmacodynamic
Drug Interactions
- What are the mechanisms of drug interactions?
- Give examples of Paramceutical mechanism.
- Pharmaceutical
Pharmacokinetic
Pharmacodynamic
- – Phenytoin and dextrose
– calcium salts and sodium bicarbonate
Drug Interactions
mechanisms of drug interactions - Pharmacokinetic
Outline how the following can result in mechanisms for drug interaction
- Absorption
- Distribution
- Metabolism
- Excretion
- pH, chelation, rate of gastric emptying
- protein binding
- Phase 1, Phase 2
- pH, competition
Drug Interactions
mechanisms of drug interactions - Pharmacokinetic
1. Absorption
What are the causal mechanisms associated with absorption?
- pH
- ?significance
- Surface area
- activated charcoal
- Chelation
- tetracyclines and calcium
- Gastric emptying
- metoclopramide
Drug Interactions
mechanisms of drug interactions - Pharmacokinetic
2. Distribution
What are the causal mechanisms associated with distribution?
-
Displacement from plasma protein binding sites
- theoretical effect for highly protein bound drugs, e.g.warfarin & phenytoin but as system is dynamic: increased free drug => increased clearance
- Displacement from tissue binding sites
- digoxin level with amiodarone therapy
Drug Interactions
mechanisms of drug interactions - Pharmacokinetic
2. Metabolism
Enzyme inducers - (PC BRAGS)
What are the enzyme inducers?
Increased synthesis or decreased breakdown of CYP isoenzymes
- Phenytoin
- Carbamazepine / Cigs
- Barbituates (Brussel Sprouts)
- Rifampicin
- Alcohol
- Glucocorticoids
- St John’s Wort
Enzyme Inhibitors
What are the Substrates and Inhibitors (1) for each of the following CYP450 Isoenzymes?
- 1A2
- 2C9
- 2D6
- 3A4
- 3A4
- Theophylline
- Ciprofloxacin
- S - Warfarin
- Amiodarone
- Oxycodone
- Fluoxetine
- Verapamil
- -azoles
- Erythromycin
- Erythromycin
An enzyme inhibitor is a molecule that binds to an enzymeand decreases its activity. Since blocking an enzyme’s activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors.

Drug Interactions
mechanisms of drug interactions - Pharmacokinetic
4. Excretion
What are the causal mechanisms of drug interactions associted with excretion?
-
Inhibition of renal tubular secretion
- probenecid - decreased secretion of penicillins
- verapamil - decreased secretion of digoxin
- salicylates - decreased methotrexate
-
Increased renal tubular absorption
- diuretics decrease sodium reabsorption producing a compensatory rise in lithium reabsorption
-
Reduced renal tubular absorption
- change in pH can alter reabsorption of drugs, e.g., alkalinization of urine in salicylate overdose
Drug Interactions
mechanisms of drug interactions - Pharmacodynamic interactions
How can these bring about drug interactions?
-
Receptor interactions
- agonists, antagonists and partial agonists
-
Physiological interactions
- potentiation, summation, antagonism

Drug Interactions
mechanisms of drug interactions - Pharmacodynamic interactions
Receptor Interactions
Give examples of receptor agonists and antagonists
- Salbutamol and Propranolol
- Morphine and naloxone
- Dopamine and chlorpromazine
Drug Interactions
mechanisms of drug interactions - Pharmacodynamic interactions
Physiological Interactions
Give examples of receptor agonists and antagonists
- Alcohol and other sedatives => increased sedation
- Beta blockers and calcium antagonists => increased antihypertensive effect
- NSAIDS decrease antihypertensive effect of ACE inhibitors
- NSAIDS and corticosteroids => increase risk of peptic ulcer
Common important interactions (1)
Warfarin
- What is it’s effect increased by?
- What is its effect decreased by?
- amiodarone, cimetidine, ciproflxacin, clarithromycin, erythromycin, fluconazole, ketoconazole, mefenamic acid, sulphonamides
- barbiturates, carbamazepine, phenytoin, rifampicin
Common important interactions (2)
Theophylline
- What is it’s effect increased by?
amiodarone, ciprofloxacin, cimetidine, clarithromycin, erythromycin, fluconazole, ketoconazole, sulphonamides
(enzyme inhibitors)
Common important interactions (3)
Digoxin
- What is it’s effect increased by?
amiodarone, diltiazem, verapamil,
diuretics
Outline the potential for disaster in drug interactions
How are prescribing skills regulated?

National Prescribing Skills Assessment

Preventing adverse drug interactions
How can these be prevented?
- Be aware of common interactions
- Use drugs only when truly indicated
- Check BNF before you prescribe any drug but especially if patient is on a drug with a narrow therapeutic range


