Session 8 Flashcards
(22 cards)
To consider the methods by which infections can spread
[*] Many (all?) infections are transmissible
- From a non-human source to humans
- From person to person either directly or indirectly
[*] Common Source examples
- Food/water e.g. good poisoning organisms.
- Environmental e.g Legionella pneumophilia due to badly set up air conditioning (but organism is not transmissible from person to person)
- Animals e.g. rabies

Describe Person to Person Direct Transmission
- Influenza
- Norovirus
- Neisseria gonorrhoea
- Could potentially lead to epidemics or pandemics

Describe Person to Person Indirect Transmission
[*] Person to Person Indirect Examples (normally via a vector)
- Mosquitos: malaria, dengue
- Cats: toxoplasmosis
- Ticks: Lyme disease, spotted fevers
Define Endemic Disease, Outbreak, Epidemic and Pandemic
[*] Endemic disease: the usual background rate (normal day to day, year to year level)
[*] Outbreak: two or more cases linked in time and place (you see more cases than you expect to see; number varies depending on each disease)
[*] Epidemic: a rate of infection greater than the usual background rate (many people infected e.g. influenza epidemic – compared to endemic disease)
[*] Pandemic: very high rate of infection spreading across many regions, countries, continents
Describe the Basic Reproduction Number
- R(0): the average number of cases one case generates over the course of its infectious period, in an otherwise uninfected, non-immune population (e.g. due to person to person transmission in the case of influenza)
- If R(0) >1 => increase in cases
- If R(0) = 1 => stable number of cases (‘stable disease’)
- If R(0) <1 => decrease in cases (logically eventually the disease should go to extinction)
- Diseases such as Measles or Pertussis with high R(0) numbers are extremely contagious.
- In comparison influenza with a R(0) of 2-3 has a relatively poor number of secondary cases.

What are the reasons for epidemics, pandemics and outbreaks?
- New pathogen (antigens, virulence factors, antibacterial resistance). Hosts do not have any immunity – such as in the 2009 swine flu from Mexico. This could also work the other way – people migrating to a new area where a pathogen already exists but a human population has never resided before so no one has caught the bug and developed immunity.
- New hosts (non-immunes, healthcare effects): people coming into a new population do not have any immunity e.g. when babies are born, it extremely rare to see cases of Neisseria meningitis within the first 3 bodies because maternal antibodies have crossed the placenta and protect the baby. Peak of infection is between 3 months and 3 years are patients are exposed to different strains => hopefully giving them lifelong immunity.
- New practice (social, healthcare): e.g. when air conditioning was first introduced, legionella pneumophilia was inadvertently introduced into the air. These days water and air are kept separate. Surgery is becoming more commonplace – initially the rate of surgical site infections was very high.

How does infectious dose determine transmissibility?
Infectious dose: Number of microorganisms required to cause infection. Varies by:
- Microorganism
- Presentation of microorganism (what it’s contained in e.g. food, water, aerosol, droplet)
- Immunity of potential host (how susceptible is the host? Consider general health, specific immunity etc)
ID(50): the number of microorganisms required to infect 50% of the population
E.coli 0157:h7 strain has a very low infectious dose – causes haemolysis and renal failure – potentially fatal cause of food poisoning

Describe epidemic curves
[*] Epidemic Curves are used when describing large outbreaks.
NB: small scale outbreaks have a stochastic nature (random, very variable) e.g. in a nursing home or in a family so it is difficult to tell whether intervention worked or whether it was due to random chance.

What are possible interventions?
Pathogen (+ vector)
- Reduction
- Eradication
- Examples: antibacterials including disinfectants, decontamination (cleaning environment), sterilisation (e.g. of surgical instruments).
- Reducing/eradicating vector could be done by eliminating vector breeding sites (e.g. marshlands where mosquitos breed)
Patient
- Improved health (nutrition, medical treatment)
- Improved Immunity: Passive e.g. maternal antibody, intravenous/intramuscular immunoglobulins (antibodies from pooled blood donors who have immunity – short lived protection) and Active e.g. vaccination (giving antigen in a form that doesn’t cause disease)
- Herd Immunity: the idea that you can protect some part of the population even if they haven’t been vaccinated by ensuring a sufficient number of people have been vaccinated. Vaccinated people develop immunity and stop transmission from infected person.
Practice
- Behavioural change => reduce probability of infection transmission
- Avoidance of pathogen or its vector: Geographic “Don’t go there”; Protective clothing and equipment (long sleeves and trousers against mosquito bites, personal protective equipment in hospitals such as gowns, gloves, masks); Behavioural (safe sex, safe disposal of sharps)
Place
- Environmental engineering e.g. single-room toilets, keeping drinking water separate from sewage, making alcohol gel available in all the corridors etc
- Safe water
- Safe air
- Good quality housing
- Well designed healthcare facilities (crowding increases risk of infection!)

What are the consequences of infection control?
[*] Good consequences of control:
- Decreased incidence or elimination of disease/organism:
- Smallpox (extinct)
- Polio (threatened with extinction)
- Dracunculiasis (parasitic guinea worm) (threatened with extinction)
[*] Bad consequences of control:
- Decreased exposure to pathogen => decreased immune stimulus => decreased antibody => increased susceptibles => outbreak
- Later average age of exposure => increased severity (the older you are when you get it, the more ill you become) e.g.
Polio
Hepatitis A and Chicken Pox (risk of pneumonitis) can be potentially fatal in adults
Congenital rubella syndrome: pregnant mothers who get infected in the first three months could have babies born with congenital rubella syndrome (severe defects/disease)
What are the principles of surveillance?
What is happening now?
- Here?
- Elsewhere?
What might happen?
Appreciate the consequences of antimicrobial resistance
[*] Antimicrobial use drives antimicrobial resistance (which can be spread from person to person e.g. MRSA)
[*] Resistance is effectively reversible
[*] Antimicrobial development is stalled (no new antibiotic classes emerging)
[*] Consequences of antimicrobial resistance
- Treatment failure ‘untreatable infection’
- Prophylaxis failure – ability to prefevent infection in patients undergoing surgical operations, leukaemia or other immunosuppressing treatments => increased susceptibility to infection
- Economic costs: new antibiotics are more expensive (e.g. to treat MRSA can cost ~£1—per person per day)
- Resistance => inappropriate treatment => increased mortality + increased economic cost

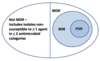
What does MDR, XDR and PDR mean?
- MDR (multi-drug resistant): non-susceptibility to at least one agent in three or more antimicrobial categories (agents not releated)
- XDR (extensively drug resistant): Non-susceptibility to at least one agent in all but two or fewer antimicrobial categories
- PDR (pan-drug resistant): non-susceptibility to all agents in all antimicrobial categories
What is Antibiotic Stewardship?
[*] ISDA definition: Coordinated interventions designed to improve and measure the appropriate use of antimicrobials by promoting the selection of the optimal antimicrobial drug regimen, dose, duration of therapy and route of administration. Antimicrobial stewards seek to achieve optimal clinical outcomes related to antimicrobial use, minimize toxicity and other adverse effects, reduce the costs of healthcare for infections and limit the selection for antimicrobial resistant strains.
- Appropriate use of antimicrobials
- Optimal clinical outcomes
- Minimize toxicity and other adverse events (such as the promotion of Clostridium difficile)
- Reduce the costs of healthcare for infections
- Limit the selection for antimicrobial resistant strains
What are the elements of an antimicrobal stewardship programme?
- Multidisciplinary team and relationships to other quality/safety teams
- Surveillance
- Process measures (measuring what you do)
- Outcome measures (measuring what consequences you get as a result of what you do)
- Interventions
- Persuasive
- Restrictive
Structural
What makes up an MDT team?
[*] Multi-disciplinary team
- Medical microbiologist / infectious diseases physician
- Antimicrobial pharmacist
- Infection control nurse
- Hospital epidemiologist
- Information specialist
- NB: some jobs are rare in the UK
[*] Antimicrobial stewardship overlaps with infection prevention and environmental decontamination (keeping hospital clean)

What are the stewardship intervention types?
Persuasive (persuading people to do the right thing)
- Education
- Consensus
- Opinion leaders
- Reminders
- Audit
- Feedback
Restrictive: preventing clinicians from prescribing certain drugs or limiting the antibiotics/doses clinicians can prescribe
- Restricted susceptibility reporting
- Formulary restriction
- Prior authorisation
- Automatic stop orders (e.g. max 5 days)
Structural
- Computerised records
- Rapid lab tests (to reduce the time a doctor has to wait to find out results – to reduce the use of empirical antibiotics)
- Expert systems (computer based)
- Quality monitoring e.g. the Cochrane project
Describe Process Measures
[*] Process Measures: measuring antibacterial use
- Quantity: e.g. defined daily doses/1000 bed days
- Antibacterial classes
- Appropriateness: adherence to guidelines
- Over time in same institution
- Benchmarking against other institutions
- (Comparing ward to ward or hospital to hospital)
Describe Outcome Measures
- Patient outcomes
- Emergence of resistance
- Clostridium difficile infection rate
What are the requirements for successful stewardship?
- Long term confirmed and appropriate resources (appropriate staff availability, requires a lot of commitment)
- Hospital leadership support and delegated authority to challenge/change inappropriate antimicrobial therapy
- Integration into organisational patient safety and quality of care structure and processes (you need a centrally embedded system)
- Evidence suggests stewardship is effective

Compare restrictive versus persuasive outcomes
Evidence suggests both are effective (no significant difference between intervention types) over time however Restrictive may be more effective over persuasive in the first 6 months.
Describe unintended consequences and outstanding questions
[*] Interventions intended to decrease unnecessary antibiotic prescribing
- Outcome: Risk of mortality for intervention versus control; Size of Effect: reduced death rate
- Outcome: Difference (in days)I n length of stay for intervention versus control; Size of Effect: reduced length of stay
- Outcome: Risk of readmission for intervention versus control; Size of effect – slight increase in risk of readmission (slightly significant)
[*] Some outstanding questions
- Is it possible to achieve multiple objectives at the same time?
- Why do doctors prescribe antibiotics? One reason is regret avoidance – they don’t want to risk missing an infection. However they are increasing the risk of antimicrobial resistance.
- Is there an optimal antimicrobial formulary? (Need to consider for each individual organisation)
- How best to maintain long-term sustainability of stewardship impact? (limited budget, other problems such 4-hour windows in A&E etc)


