Respiratory pathology Flashcards
What is COPD?
- progressive airway obstruction which does not change markedly over several months
- characterised by persistant airflow obstruction
- poorly reversible + usually progressive
- obstructive element important; it’s obstruction to airflow which causes the disabling symptoms of breathlessness + impairs QoL
The term COPD has replaced the old terms chronic bronchitis and emphysema. What are these two things?
- chronic bronchitis - defined clinically as ‘cough productive of sputum for 3 consecutive months for 2 consecutive years which cannot be attributed to other cardiac or pulmonary disease’
- emphysema - defined pathologically as ‘permanent dilatation of airways distal to terminal bronchiole’. It is an ‘apparent’ dilatation of airspaces but is, in fact, due to destruction of alveolar walls
Most COPD patients have features of both chronic bronchitis + emphysema since they share a common trigger (smoking).
The airflow obstruction is the result of damage to both small conducting airways and alveoli. What factor almost always causes the damage?
- tobacco smoking
- other important factors: occupation eg. those associated w/ dust (mining), a1-antitrypsin deficiency
- important to realise that only a minority of smokers develop COPD
- reason for this unknown, but evidence suggests that inflammatory response is amplified in susceptible people
All the airways throughout the respiratory tract are damaged by smoking, although the specific effects at each level are different.
What happens at the bronchi with smoking?
- hyplerplasia of mucus-producing glands in submucosa
- hyperplasia of goblet cells on surface epithelium
- leads to increased sputum production
What happens at the small airways with smoking?
- chronic inflammation
- healing by fibrosis
- stenosis of the airways
What effect does smoking have at the respiratory bronchioles?
Destruction of the walls with loss of elastic tissue but without significant fibrosis -> airway dilatation -> emphysema
Destruction has 2 major effects:
- loss of pulmonary SA for gas exchange -> hypoxia
- loss of elastic tissue of terminal airways -> loss of natural recoil of lungs -> contributes to reduction in airflow on expiration ie. airflow obstruction
Remember: in normal lungs the elastic recoil acts to collapse the lung and is opposed by negative intrapleural pressure which maintains lung expansion
What is the protease/antiprotease hypothesis?
- may account for lung destruction in emphysema
- smoking causes inc # of activated neutrophils in lung
- where they release protease enzymes (elastase)
- in adddition, smoking inhibits the lung’s natural protease inhibitor enzymes eg. a1-antitrypsin
- therefore, large amounts of active elastase enters lung interstitium
- binds to + degrades elastin
- results in destruction + enlargement of distal airspaces

The clinical effects of smoking vary between individuals, some may develop smoker’s cough + inc sputum production yet remain relatively free from obstruction + breathlessness and vice versa.
What is the clinical presentation of COPD?
- symptoms have an insidious onset
- earliest symptom in natural history of COPD = cough + sputum
- reflects involvement of larger airways
- susceptible individuals continue to smoke? -> small airways become inc obstructed -> sudden SoBoe
- advanced disease -> breathlessness occurs upon minimal exertion and then at rest
- death in COPD is usually from bronchopneumonia, resp or heart failure
What is the role of spirometry in the diagnosis of COPD?
spirometry confirms diagnosis of COPD by demonstrating airflow obstruction
Describe an acute exacerbation of COPD
- sudden, sustained worsening in pt’s symptoms
- beyond their normal day-day variation
- eg. worsening breathless + cough, inc sputum
- may require a change in treatment
- infection (bacterial or viral) = most common cause of an acute exacerbation of COPD
- other less common causes incl pneumothorax, PE, LVF, lung carcinoma
How is an infective exacerbation of COPD different from pneumonia?
- the airways are focus of infection in an infective exacerbation
- in pneumonia, infection is centred on the alveoli

COPD is the most common cause of cor pulmonale (right heart failure due to lung disease).
What other changes/complications can COPD induce in the pulmonary circulation?
- emphysema -> loss of pulmonary arterioles + capillaries
- chronic hypoxia -> pulmonary artery vasoconstriction
- chronic hypoxia -> inc EPO prod by kidney -> inc RBC prod -> inc blood viscosity
All these changes contribute to gradual development of pulmonary hypertension.
Initially, the right ventricle undergoes compensatory RV hypertrophy but eventually the RV decompensates and right heart failure ensues.

What is pneumonia?
- inflammation of the lung parenchyma (ie alveolar spaces)
- due to an infective agent
What is the pathological classification of pneumonia?
lobar vs bronchopneumonia
-
BRONCHOPNEUMONIA
- widespread patchy inflammation centred on airways
- often bilateral
- patchy areas of consolidation
- bronchi containing acute inflammatory exudate
- also upper lobe emphysema
-
LOBAR PNEUMONIA
- diffuse inflammation affecting an entire lobe/lobes
- photo - entire lobe, paler than other
- consolidation due to accum of acute inflammatory exudate within alveoli
- abrupt demarcation at interlobar fissure
This classification largely was based on macroscopic exam of lungs at autopsy in pts w/ florid pneumonias in a pre-antibiotic era. Problem - difficult to apply in most cases as patterns overlap + classical picture is extremely blurred by modern day abx therapy.
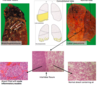
What does consolidation refer to?
- on CXR - refers to replacement of air in alveoli by fluid or other material, with preservation of underlying alveolar architecture
- in case of pneumonia, air is replaced by acute inflammatory exudate
- there is no destruction of underlying architecture
A more clinically relevant classification is based on circumstances surrounding development of pneumonia. These subdivisions are more useful bc each type of pneumonia is associated w/ a particular group of likely pathogens.
Pts can be easily placed into a category + the relevant antimicrobial therapy can be prescribed.
What organisms cause community-acquired pneumonia?
- strep. pneumoniae (pneumococcus) single most common cause (-> mild + severe pneumonia)
- influenza + other viruses
- chlamydia pneumoniae/psittaci
- mycoplasma pneumoniae
- legionella pneumonia (-> severe pneumonia)
- haemophilus influenzae
- s. aureus (-> severe pneumonia)
What is the CURB-65 severity score for community-acquired pneumonia?
CURB-65 score used to assess the severity of CAP, it’s been validated for predicting mortality
each risk factor scores one point, for a max score of 5:
- Confusion of new onset (AMT ≤8)
- Urea >7mmol/L
- Resp rate ≥30
- BP <90 systolic or ≤60 diastolic
- 65 years or older
The risk of death increases as the score increases - helps in deciding which pts require hospital admission
Hospital-acquired pneumonia occurs 2 days or more after admission to hospital.
What organisms are responsible for this?
Gram negative bacteria are responsible for ~60% of cases
- Klebsiella
- E. Coli
- Pseudomonas
S. aureus and S. pneumoniae are also important causes
What is aspiration pneumonia due to?
- pneumonia due to aspiration
- particularly a risk in:
- intoxicated patients
- acute stroke patients w/ impaired swallowing
- septic patients w/ reduced consciousness
Aspiration pneumonias are often mixed infections including anaerobes
Conventional respiratory pathogens are still common but generally the infection is more severe, with pneumonia in the immunocompromised.
In addition, they are susceptible to less virulent organisms, such as?
- fungi eg. pneumocystitis, candida, aspergillus
- mycobacterial infection eg. M. tuberculosis or atypical mycobacteria
- viruses eg. CMV, HSV
What happens in diffuse parenchymal lung diseases (=intersitital lung diseases)?
- large group of conditions characterised by inflammation centres on the interstitium of alveolar walls
- interstitium becomes expanded by inflammatory cell infiltrate (‘pneumonitis’ or ‘alveolitis’)
- impairs gas exchange + causes breathlessness
- episodes of alveolitis may be followed by complete regeneration without residual damage to alveoli
With interstitial lung disease where most inflammation may completely regenerate, sometime the inflammation is followed by repair with scarring.
What happens here?
- macrophages release fibrogenic cytokines
- stimulate fibroblasts in interstitium
- secrete collagen (scar tissue)
- thickened alveolar walls are ineffective at gas exchange
- resulting in worsening breathlessnes

What is meant by the terms ‘pneumonitis’, ‘alveolitis’ and ‘pneumonia’?
- pneumonitis - inflammation of lung parenchyma ie. the alveoli (alveolitis is an alternative name) - usually due to non-infective causes. Inflammation is limited to interstitium.
- pneumonia - inflammation of the lung parenchyma due to an infective agent - characterised by consolidation - acute inflammatory exudate filling alveolar spaces.
There are 200+ DPLDs. They can be caused by anything which sets up chronic inflammation within the interstitial space of alveolar walls.
What are the 5 basic categories that can divide up the causes?
- unknown cause (idiopathic intersitital lung disease)
- pneumoconioses (inhaled inorganic/mineral dusts), eg. coal dust, silica, asbestos
- extrinsic allergic alveolitis (inhaled organic) eg. bird fancier lung, farmer’s lung
- side-effects of treatment - eg. therapeutic chest radiation, certain drugs (amiodarone)
- multisystem diseases involving lung eg. sarcoid, SLE, RA, scleroderma






