Gynae + testicular pathology Flashcards
What is the epithelium type of the cervix?
- endocervix lined by columnar (glandular) epithelium
- ectocervix lined by squamous epithelium
the two types of cells meet at the squamocolumnar junction

How does the cervix histology change during puberty and pregnancy?
During puberty and pregnancy, hormonally-induced eversion of cervix occurs: lower pH of vagina results in formation of a physiological transformation zone (TZ):
- the columnar epithelium undergoes physiological metaplasia to tougher + more resistant squamous epithelium
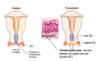
Following the physiological metaplasia, what other changes can occur in the transformation zone?
- cells undergoing metaplasia are predisposed to develop dysplasia
- so it is in transformation zone that virtually all cervical dysplasia arises
- in the cervix, the agent inducing dysplasia is HPV
- dysplasia occuring in the cervix is called cervical intraepithelial neoplasia (CIN) - usually asymptomatic
- left untreated, CIN may progress to invasive squamous cell carcinoma

How likely is CIN progression to invasive carcinoma?
- ~ 11% of CIN 1 will progress to CIN 3 (other 89% stays as CIN 1 or regresses)
- ~ 12% of CIN 3 will progress to invasive cancer (other 88% stay as CIN 3 or regresses)
What is the major risk factor for development of CIN and cervical cancer?
- persistent HPV infection
- over 99.5% of cervical cancers associated w/ high risk of HPV infection
- more than 130 HPV genotypes identified
- sexually transmitted
HPV infections of the genital tract have been subclassified into those associated w/ benign (low risk) and malignant (high risk) genital tract disease.
What are the high risk subtypes?
- HPV types 16 + 18
- associated w/ 70% of cervical cancers
What are the low-risk HPV subtypes?
- HPV types 6 + 11
- subtypes associated w/ anogenital warts (condyloma acuminata) but not CIN and cervical cancer
What is the pathophysiology of the HPV virus and how it infects?
- HPV viral DNA integrates into host cell DNA in the cervical squamous epithelium
- the virus preferentially infects cells in the TZ since these cells are undergoing metaplasia and altered gene expression
- the viral E6 + E7 gene products interact with and inhibit tumour suppressor gene products: P53 and retinoblastoma protein
- these proteins are important for cell cycle control + apoptosis
- inactivation of these genes -> uncontrolled cell proliferation
- HPV is a prerequisite for cervical cancer but only small proportion of HPV infections progress to either high-grade CIN or cancer
- progression to malignancy requires one or more cofactors eg. smoking, immunosuppression
Cervical cancer is the most common cause of cancer in women aged 18-35yrs. How does cervical cancer usually present?
- vaginal bleeding
- most commonly post-coital bleeding
- post-coital bleeding is due to cervical cancer until proven otherwise
- there may be an offensive vaginal discharge
- pain is not an early feature
- speculum exam usually reveals an ulcer or a mass on cervix
Following examination, what investigations can be carried out for suspected cervical cancer?
- biopsy -> to confirm diagnosis, give the type and grade
- staging -> examination under anaesthesia, abdo/pelvis CT
- staged using FIGO system
NB. a smear is a screening test for asymptomatic women and so is not appropriate in a symptomatic woman
What is the pathology/type of most cervical cancers?
- invasive squamous cell carcinomas (80%)
- precursor lesion is CIN
What are the remaining 20% of cervical cancers?
- adenocarcinomas
- arising from endocervical epithelium in cervix
- high risk HPV also important in causing cervical adenocarcinoma
- precursor lesion is CGIN (cervical glandular intraepithelial neoplasia)
CGIN beyond scope of T year syllabus
Describe the cervical screening programme
- to detect + treat premalignant lesions (ie. CIN)
- therefore to reduce incidence + mortality of squamous cell carcinoma
- cervical smears are basis of programme
- women screened every 3 years from 25-49yrs and every 5yrs from 50-64yrs
- test involves opening up vagina w/ speculum and using a brush to take sample of cells from the transformation zone
- the brush, where cells are lodged, rinsed directly into preservative fluid
- this is sent to cytology lab for prep as a slide
- stained slides scrutinised for squamous epithelial cells showing dyskaryosis

What is dyskaryosis?
- refers to abnormalities of the cell nucleus
- eg an irregular shape, an increased size
- term that is only used in cervical smear reports
- dyskaryosis is graded depending on severity of nuclear abnormalities:
- low grade - mild
- high grade - moderate or severe

What is the difference between dyskaryosis and CIN regarding terminology?
- dyskaryosis - diagnosis rendered on cervical smear (screening)
- CIN - diagnosis only rendered on cervical biopsy (gold-standard)
A smear showing dyskaryosis is a good predictor of the presence of CIN in the cervix. What dyskaryosis grades correlate with the level of CIN?
- low grade (mild) dyskaryosis - CIN 1
- high grade (mod) dyskaryosis - CIN 2
- high grade (severe) dyskaryosis - CIN 3
These are all PREDICTIONS - like all screening tests, the cervical smear is not perfect + sometimes is wrong. A definite diagnosis of CIN can only be made on biopsy (or LLETZ) taken at colposcopy.
What is meant by ‘borderline nuclear change’?
- a reporting category which is best thought of as a holding category
- used when the pathologist is uncertain whether the smear is normal or shows dyskaryosis
- does not correspond to a particular disease process
- borderline category is necessary bc the cervical smear is an imperfect screening test which doesn’t always give a clear result
What are the national guidelines for management of abnormal cervical smears?
- high-grade dyskaryosis (mod/severe) -> refer pt to colposcopy
-
low-grade dyskaryosis (mild) -> HPV testing using PCR performed on smear sample to see if high risk HPV subtypes (16+18) are present in sample:
- high-risk HPV positive -> refer pt to colposcopy
- high-risk HPV negative -> return pt to routine recall (3-5yrs)
- borderline nuclear change managed in same way as low-grade dyskaryosis (ie HPV triage)
Why is it reasonable to “return to routine recall” if the high-risk-HPV test is negative?
- in absence of high-risk HPV, CIN is very very unlikely
- therefore it is safe to assume the ‘mild dyskaryosis’ seen in smear is actually a false positive
- reflects imperfections of the screening test
What happens at colposcopy?
- cervix exposed using a speculum
- colposcopist carefully examines cervix using colposcope
- both before and after application of acetic acid
- certain abnormal colposcopic appearances are associated w/ CIN eg. acetowhite epithelium
- however, CIN is a histological diagnosis so biopsy required to confirm diagnosis

What is the management of CIN?
- CIN 1 - observation + regular follow-up smears
-
CIN 2 + 3 - excision of transformation zone w/ cutting diathermy under local anaesthesia - ‘large loop exicison of transformaton zone’ (LLETZ)
- specimen sent to lab to be carefully examined histologically by pathologist
- pts who have had a LLETZ for CIN are offered a repeat smear + high risk-HPV testing 6 months later

What is the endometrium composed of?
- endometrial glands
- endometrial stroma
- in normal endometrium, the glands are widely spaced in the stroma

What is endometrial hyperplasia?
- increase in the number of endometrial glands relative to the endometrial stroma
- glands become more closely packed
- results in a thickening of endometrium
- can be seen at hysteroscopy or on imaging (transvaginal ultrasound)

How does endometrial hyperplasia usually present?
- abnormal vaginal bleeding
- intermenstrual, polymenorrhoea (unusually frequent periods)
- or postmenopausal











