Renal Flashcards
Renal Functions
Overview
-
Excretion:
- metabolic wastes and compounds excreted in the urine
-
Homeostasis:
- water and electrolyte balance
- extracellular fluid volume
- plasma osmolarity
-
RBC production:
- secretes erythropoietin (EPO)
-
Regulation of vascular resistance:
- Renin secreted by juxtaglomerular apparatus cells
- Initiates RAAS controlling vascular resistance
-
Regulation of acid-base balance
- Secretes or absorbs acids and bases
-
Vit D production
- Calcitriol
-
Gluconeogenesis
- Contributes during prolonged fasting
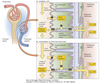
Cortex
Outer part of the kidney which contains:
- Bowmen’s capsules
- glomeruli
- proximal convoluted tubules
- cortical portions of loop of Henle
- distal convoluted tubules
- cortical collecting tubules
- tubules and microvasculature intertwined in a random fashion
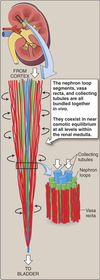
Medulla
Inner part of the kidney composed of pyramids.
Contains:
- medullary portions of loop of Henle
- medullary collecting tubules
- collecting ducts
- tubules and blood vessels arranged in parallel
Calyces
- drain into the renal pelvis
- renal pelvis forms the head of the ureter
- ureter sends urine to the urinary bladder
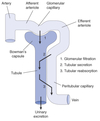
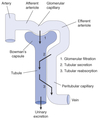
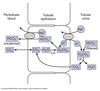
Nephron Structure
Functional unit of the kidney.
-
Bowman’s capsule is the start of the nephron
- Blood enters via the afferent arteriole
- Moves into the glomerular capillaries
- Exits via the efferent arteriole
- Filtrate enters Bowman’s space
-
Proximal tubule
- Proximal convoluted tubule (PCT) ⇒ cortex
- Proximal straight tubule (PST) ⇒ medulla
-
Loop of Henle:
- Descending thin limb (DTL)
- Ascending thin limb (ATL)
- Thick ascending limb (TAL)
- Distal convoluted tubule (DCT)
- Connecting tubule
- Cortical and medullary collecting ducts
- Calyx → renal pelvis → ureter → urinary bladder

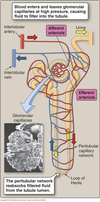
Peritubular Capillaries
- Surrounds the nephron tubule
- Provides O2 and nutrients to tubules.
- Carries away fluid and electrolytes reabsorbed by tubules.
Superificial Nephrons
- Receives ~ 90% of renal blood supply
- Major site of fluid and electrolyte reabsorption
- Short loops of Henle that do not reach the inner medulla
Juxtamedullary Nephrons
- Receive ~ 10% renal blood supply
- Very long loops of Henle that go deep into inner medulla
- Peritubular capillaries forms a long looping vascular network ⇒ vasa recta
- Creates osmotic gradients that concentrate urine
Filtration
Process by which water and solutes leave the glomerular capillaries and enter Bowman’s space.
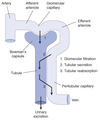
Secretion
Process where substances are transported from the tubular epithelial cells into the nephron lumen.
Substances can be synthesized in the epithelial cells or obtained from surrounding interstitial space.
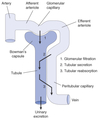
Reabsorption
Process by which substances in the lumen cross the epithelial barrier into the interstitial space where they can be absorbed by peritubular capillaries.
Excretion
The exit of substances from the body via the urine.
Reabsorption & Secretion
Overview
-
Proximal Convoluted Tubule (PCT)
- Isomolar reabsorption of ~ 70% of filtered water and solute
- Major role in reclaiming salt and water needed to maintain ECF.
- Carrier-mediated Na+ transport.
-
Loops of Henle (LoH)
- Carrier-mediated NaCl reabsorption by the water impermeable thick ascending limb.
- Generation of corticomedullary concentration gradient.
-
Distal Convoluted Tubule (DCT)
- Carrier-mediated reabsorption of NaCl.
- Water impermeable.
- Hormone-modulated Ca++ reabsorption.
-
Connecting Tubule (CNT)
- Both carrier-mediated and ion channel (hormone-regulated) Na+ reabsorption.
- Hormone-regulated Ca++ reabsorption.
- Arginine vasopressin (AVP)-responsive water permeability.
-
Collecting Tubules and Collecting Ducts
- Different cell types for Na+ reabsorption and H+ secretion.
- Hormone regulated ion channel Na+ reabsorption.
- Hormone modulated K+ secretion.
- Hormone modulated H+ secretion.
- Arginine vasopressin (AVP)-responsive water permeability.
- Urea transport.

Renal Clearance
The volume of plasma the kidneys completely clear of a substance per unit time.

Glomerular Filtration Rate
(GFR)
The rate at which the kidney’s are filtering.
- Usually corrected to body surface area of 1.73 m2.
- Normal:
- 125 ± 15 ml/min for young adult males
- 110 ± 15 ml/min for young adult females
- GFR declines after age 45-50
- Typically 30-40% lower by age 80
- Used to determine:
- Overall kidney function
- How much a substance is filtered per unit time
- Is a substance reabsorbed or secreted
- What percentage of a filtered substance is reabsorbed
- Assesses overall renal glomerular and net renal tubular function
Inulin Clearance & GFR
Insulin is the gold standard for measuring GFR.
- cleared from the plasma by filtration only
- freely filtered by the glomeruli
- not reabsorbed or secreted by tubular cells
- not created or metabolized by the kidneys
Amount of insulin filtered = amount excreted in the urine.
Clearance of inulin = GFR.
Renal
Mass Balance
-
One entrance ⇒ renal artery.
- 20% of plasma filtered into Bowman’s space
- Remainder enters efferent arteriole ⇒ renal vein
-
Two exits ⇒ renal vein and ureter.
- Reabsorbed fluids/solutes ⇒ renal vein
- Substances not reabsorbed enters ureter ⇒ urine
- Substances secreted into tubules ⇒ ureter ⇒ urine
What enters the kidney equals what exits:
Amountfiltered + Amountunfiltered = Amounturine + Amountvein
Amount filtered is GFR x plasma concentration:
Amountfiltered = GFR x Px
Amount excreted is urine flow rate x urine concentration:
Amountx-excreted = ⩒ x Ux
Para-aminohippuric Acid
(PAH)
Used at low concentrations for determination of renal plasma flow rate (RPF) due to these characteristics:
- freely filtered by glomeruli
- any PAH not filtered is transported by tubular cells from peritubular capillaries into urine
- is not synthesized or metabolized by the kidney
* PAH is not naturally-occuring and must be administered by continuous IV.
Renal Plasma Flow Rate
(RPF)
The rate of plama flow through the glomeruli in ml/min.
PAH usually used in RPF determinations.
CPAH = (UPAH x ⩒) / PPAH = RPF
Renal Blood Flow
(RBF)
The amount of blood flow through the kidneys in ml/min.
Calculated from the renal plasma flow rate (RPF) using the hematocrit (Hct) of the blood.
Since Hct is the fraction of blood volume made up of RBC, the remainder is plasma.

Filtration Fraction
(FF)
How much plasma is filtered through the glomeruli compared to how much is not.
Amount of plasma entering the glomerulus = RPF.
Amount of plasma filtered = GFR.
FF = GFR / RPF
Typical filtration fraction ~ 20%.
Applications of Clearance
Comparing clearance of any substance to that of inulin allows determination of overall net action of the renal system on that substance.

Creatinine Clearance
The clinical standard for estimating GFR (eGFR) utilizing empirical equations.
- Creatinine is the muscle creatine phosphate breakdown end-product.
- Daily creatinine production ∝ muscle mass.
- If renal function normal: creatinineexcreted = creatinineproduced so plasmacreatinine fairly constant.
- Very small amount of creatinine is secreted into urine so creatinine clearance slightly overestimates GFR.
- As serum creatinine doubles, GFR decreases by 50%.

Total Body Water
(TBW)
TBW ~ 60% of body weight in lean adult male.
TBW ~ 50% of body weight in lean adult female.
The higher the % body fat, the lower contribution of TBW to body weight.
2/3 of TBW is intracellular fluid (ICF).
1/3 of TBW is extracellular fluid (ECF).
~75% of ECF is interstitial fluid and ~25% is plasma.
Total amount of Na+ in the body determines ECF volume.
Kidneys control Na+ and water reabsorption independently of each other to maintain optimal volume and solute concentrations.

TBW
Ionic Compositions
ECF: High [Na+] & low [K+]
ICF: Low [Na+] & high [K+]
Distribution of Na+ and K+ due to the Na/K-ATPase.

Indicator-Dilution Technique
Compound known to evenly distribute through compartment of interest used to determine compartment volumes.
At equilibrium, plasma concentration measured.
Volume determined from:
Volume = amount of indicator / [indicator]
Measured using:
-
TBW
- heavy water (D2O)
- antipyrene
-
ECF
- impermeant ions
- Na+
- radioactive 35SO4
- inert sugars
- mannitol
- sucrose
- inulin
- impermeant ions
-
ICF
- difference between TBW and ECF
ECF
Volume Changes
Volume expansion and volume contraction refers to ECF volume changes.
Normal:
Width = volume
2/3 ICF and 1/3 ECF
Height = solute concentration as osmolatity
Osmolality of both compartments 285 mOsm/kg
When NaCl is added to the ECF, all of the NaCl remains in the ECF.

Hypo-osmolar
Volume Expansion
2 L of pure water added to ECF
- TBW increased 42 L → 44 L
- Osmolality decreased due to dilution
- Water moves ECF → ICF to maintain osmotic eq.
- 2/3 water added ends up in ICF

Iso-osmolar
Volume Expansion
2 L of isotonic saline (0.9% NaCl) added to ECF.
- TBW increases 42 L → 44 L
- Osmolality of ECF unchanged
- No net water movement
- Only ECF volume increases
- No change in osmolaity in either compartment

Hyper-osmolar
Volume Expansion
1 L of concentrated NaCl (5% NaCl) added to ECF.
- TBW increases 42 L → 43 L
- ECF osmolality increases
- Water moves ICF → ECF until osmotic gradient eliminated
- ICF volume decreases
- ECF volume increases
- Osmolality of both compartments increased

Iso-Molar Volume Contraction
- Commonly seen after viral gastroenteritis
- Water lost but kidneys maintain ECF [Na+] in normal range
- ECF volume falls
- No net water movement
- All of the volume loss from ECF
Hyper-osmolar Volume Contraction
- Lost in the desert without water
- Assume that only water is lost → TBW decreased
- ECF volume decreased → osmolality increases
- Water moves from ICF → ECF until equilibrium
- Both ECF & ICF volumes decreased
- 2/3 ICF & 1/3 ECF distribution maintained
- Osmolality in both compartents equal or greater than before
Hypo-osmolar Volume Contraction
- Seen with adrenal insufficiency
- Reduced aldosterone production
- Decreased renal absorption of Na+
- Slightly decreased water reabsorption → net water loss → reduced TBW
- ECF volume and [Na+] decreased
- Osmolality ICF > ECF
- Water moves ECF → ICF
- After equilibrium:
- TBW and ECF volumes reduced
- ICF volume increased
- Osmolality of ECF and ICF lower
Volume Expansion & Contraction
Summary Table

Glomerular Vascular Structure
Glomerular and peritubular capillaries form an arterial portal system.
- Glomerular capillaries sit in Bowman’s capsule.
- Peritubular capiilaries warps around tubules.
- Cells lining tubules reabsorb solutes/water from filtrate or secrete into them.
Renal artery ⇒ afferent arteriole ⇒ glomerular capillary bed ⇒ efferent arteriole ⇒ peritubular capillary bed ⇒ renal vein.
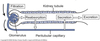
Filtration Barrier
Composed of 3 layers:
-
Capillary endothelium
- Fenestrated
- Minimally-selective openings 70-100 nm diameter
- Negative surface charge ⇒ inhibits passage of negatively charged solutes
-
Basement membrane
- Secreted by both endothelium and epithelium
-
Type IV collagen
- Inhibits passage of intermediate to large sized molecules
-
Negatively charged extracellular matrix material
- Restricts passage of negatively charged molecules
-
Epithelial layer
- Made of podocytes
- Specialized epithelial cells
- Foot processes with secondary toe-like pedicles
- Slit diaphrams between pedicles
-
Pedicles & slit diaphragms with negative surface charge
- Inhibits passage of large negatively charged molecules
- Slit diaphragms connected to contractile elements in podocytes
- Permits control over epithelial permability
- Made of podocytes
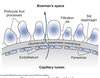
Ultrafiltration
- Filtered fluid from glomeruli enters nephron for further processing
- Glomerulus serves as molecular sieves
- water, electrolytes, and solutes with MW < 5,200 freely filtered
- Variably filtered substances with MW 6,000 to 60,000
- Permeability related to size, shape, and charge

Starling’s Law
&
Glomerular Filtration
Forces that govern fluid movement across glomerular capillary
described by Starling’s Law:
GFR = K<em>f</em>[(PGC + πBS) - (πGC - PBS)]
K<em>f</em> = filtration coefficient
PGC = hydrostatic pressure of glomerular capillary
PBS = hydrostatic pressure of Bowman’s space
πGC = oncotic pressure of glomerular capillary
πBS = oncotic pressure of Bowman’s space ⇒ 0 since proteins are not passed through the capillary
Ultrafiltration Pressure
(PUF)
Net force for fluid movement (PUF) at glomerular capillaries approximately + 10 mmHg.
Fluid pushed out of the capillary.
- PBS and πBS constant under normal conditions
-
πGC higher at efferent arteriole than afferent
- Due to ~15-20% volume loss by blood
-
Capillary hydrostatic pressure (PGC) is the main driving force for filtration
- PGC at afferent arteriole higher than other capillary beds
- At start of renal arterial portal system
-
Small pressure drop exists along the capillary
- PGC @ afferent arteriole ~ 60 mmHg
- PGC @ efferent arteriole ~ 58 mmHg
- Depends on
- aortic pressure
- renal arterial pressure
- afferent and efferent arterial resistance
- PGC at afferent arteriole higher than other capillary beds

Effects of Arteriolar Changes
Afferent arteriole
-
Constriction decreases glomerular blood flow
- Dec. PUF and GFR
-
Dilation increases glomerular blood flow
- Inc. PUF and GFR
Efferent arteriole
-
Constriction decreases glomerular blood flow
- Causes pressure to back up in capillary bed ⇒ inc PGC
- Inc. PUF and GFR
-
Dilation increased glomerular blood flow
- Causes drop in PGC
- Dec. PUF and GFR
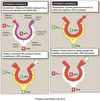
Autoregulation
Glomerular Blood Flow
Ensures that RBF and GFR remains relatively stable over a wide range of MAPs (80-180 mmHg).
Minimalizes the impact of changing arterial pressure on Na+ excretion.
1. Myogenic Response
2. Tubuloglomerular feedback (TGF)

Myogenic Response
Auto-regulatory Mechanism
- Increase in pressure results in vascular contraction to maintain a constant blood flow
- Mediated by stretch-activated cation channels
- Opens in response to increased translumenal pressure
- Depolarizes smooth muscle cells
- Elicits contraction
- Found only in afferent arterioles

Tubuloglomerular Feedback
(TGF)
Auto-regulatory mechanism unique to the kidneys.
- Mediated by the macula densa
- Part of the juxtaglomerular apparatus
- In the thick ascending limb at the transition of the LoH to the distal convoluted tubule
-
Macula densa cells monitor local Na+ and Cl- concentrations
- Increased PGC ⇒ Increased GFR ⇒ Increased NaCl delivery to macula densa
- Cells release ATP ⇒ adenosine
-
Adenosine binds to:
-
A1 purine receptors on afferent arterioles
- Vasoconstriction
- Reduces blood flow & GFR
-
A2 purine receptors on efferent arterioles
- Vasodilation
- Increases outflow
- Decreases PUF & GFR
-
A1 purine receptors on afferent arterioles
At high flow rates:
Increased NaCl delivery to macula densa ⇒ ATP ⇒ adenosine production ⇒ afferent arteriole constriction & efferent arteriole dilation AND renin release from granular cells inhibited ⇒ decreased PUF & GFR.
At low flow rates:
Decreased NaCl delivery to macula densa ⇒ no adenosine made ⇒ inhibition of renin release removed ⇒ renin starts RAAS cascade ⇒ increased PUF & GFR.

Nervous System Control
Glomerular Blood Flow
- Drop in blood pressure triggers carotid and aortic baroreceptor reflex
- SNS activation results in release of norepinephrine
- Leads to vasoconstriction
-
Efferent arterioles more sensitive to norepi
-
Mild SNS activation preferentially constricts efferent arterioles
- Results in reduced RBF maintaining GFR
-
Intense SNS activation constricts both affrent and efferent arterioles
- Reduces GFR
-
Mild SNS activation preferentially constricts efferent arterioles
- Severe hemorrhage causes prolonged constriction of renal arterioles
- Can lead to renal ischemia and failure
-
Efferent arterioles more sensitive to norepi
Hormonal Control
Glomerular Blood Flow
- Low MAP pressures triggers renin release from granular cells of JGA
- Renin converts Angiotensinogen ⇒ Angiotensin I
- ⇒ Angiotensin II by ACE in lungs
-
Ang II is a potent vasoconstrictor
- Stronger effect on efferent arteriole compared to afferent
- Net effect that GFR increases and RBF decreases
- Ang II also induces renal production of prostaglandin vasodilators PGE2 and PGI2
- PGE2 and PGI2 preferentially dilates afferent arteriole
- counterbalances constriction of afferent arteriole by Ang II
- Ensures that RBF is not decreased too much
Glomerular Blood Flow Regulation
Summary Table

Renal Transport Mechanisms
Substances can cross the renal epithelial barrier via two pathways:
-
Paracellular route
- Movement between cells in the epithelia
- Permeability depends upon tightness of intercellular junctions
- Mostly water with associated solvent drag
-
Transcelluar route
- Movement across the apical and basolateral membranes of epithelial cells
- Usually requires a channel or transporter in each membrane
- Solute and water transport depends on
- presence of channel/transport
- distribution along nephron
- location in basolateral/apical membrane
- Example aquaporins:
- Always present in proximal tubule and descending thin limb of LoH
- Controlled expression by ADH/AVP in collecting ducts

Driving Force
for
Renal Transport
-
Electrochemical Na+ gradient
- Set up by the basolateral Na/K-ATPase
- High [Na+] in lumen
- Low intracelluar [Na+]
- Transmembrane potential ~ 70 mV
- Major driving force for solute and water movement
- Drives movement of Na+ into epithelial cells
- Then pumped into interstitial space & returned to circulation
- Set up by the basolateral Na/K-ATPase
-
Transepithelial potential
- Also set up by the Na/K-ATPase
- ~ 3 mV with lumen negative
- Driving force for movement of anions via paracellular pathway
-
Osmotic gradient
- Na/K-ATPase: 3 Na+ out for every 2 K+ in from insterstitial space
- Drives water movement across epithelial barrier for water reabsorption
- Transcellular via aquaporins
-
Paracellular via intercellular junctions
- Water movement can sweep ions and small organic molecules with it ⇒ solvent drag
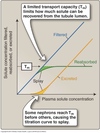
Tubular Transport Maximum
(Tm)
- Transporters exhibit saturation at high solute concentrations
- Maximal transport rate ⇒ tubular transport maximum (Tm)
- As [solute] reaches Tm, not all solute reabsorbed and some excreted in urine.
- Solutes begin appearing in urine before Tm reached ⇒ splay
- Represents heterogeneity in transporter distribution and function
- As [solute] increases, urine concentration increases
- Solutes begin appearing in urine before Tm reached ⇒ splay
Proximal Convoluted Tubule
Transport Overview
Uses both transcellular and paracellular pathways.
Tight junctions are highly permeable to H2O, Na+, K+, Cl-.
Unable to maintain Na+ and K+ gradient.
PCT reabsorption is isosmotic.
-
Reabsorbs:
- Essentially all filtered glucose and amino acids
- Largest fraction (~ 70%) of filtered:
- Na+
- K+
- Ca2+
- Cl-
- HCO3-
-
Secretes:
- Various organic cations and anions
-
Synthesizes and excretes:
-
NH3/NH4+
- Role in HCO3- generation & acid/base regulation
-
NH3/NH4+
PCT
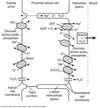
Na+ Reabsorption
70% of the Na+ that enters PCT is fully reabsorbed.
-
Transcellular
-
Basolateral
-
Na/K-ATPase
- sets up Na+ gradient
- sets up transmembrane potential
- starts process of Na+ reabsorption
- Na+/HCO3- cotransporter
-
Na/K-ATPase
-
Apical
- Various Na+ coupled transporters
- glucose
- amino acid
- phosphate
- organic acid
- NHE3 Na+/H+ exchanger
- Various Na+ coupled transporters
-
Basolateral
-
Paracellular
- Driven by the transepithelial potential (- 3 mV in lumen)
- Cl- from lumen ⇒ interstitial space
- Some reabsorbed Na+ can leak back to lumen
PCT
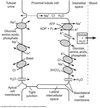
Water Reabsorption
-
Reabsorption:
-
Transcellular
- Aquaporins always present in PCT
-
Paracellular
- Movement of Na+ and other solutes creates a temporary osmotic gradient
- PCT epithelium very water permeable
- Drives water movement from lumen across epithelial layer
-
Transcellular
- Water that enters interstitial space is absorbed by peritubular capillaries.
- Capillary oncotic pressure (πPC) > capillary hydrostatic pressure (PPC)
- Water moves into the capillary
PCT
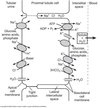
Bicarbonate Reabsorption
PCT is the main site for bicarb recovery (~80%).
- HCO3- charged & cannot diffuse across cell
- No HCO3- transporters on apical membrane
- PCT secretes H+ into lumen using NHE3 Na+/H+ exchanger
- H+ converts HCO3- into H2CO3
- Carbonic anhydrase IV converts H2CO3 to CO2 and H2O
- CO2 and H2O easily enters the cell.
- Inside, carbonic anhydrase II converts CO2 back to H+ and HCO3-.
- H+ re-enters lumen via NHE3 Na+/H+ exchanger
- HCO3<strong>-</strong> moved to interstitial space by Na+/HCO3- cotransporter.
Proximal Tubule
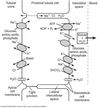
Cl- Reabsorption
-
Enters the cell (apical)
-
Paracellular
- Throughout PCT
- Driven by postive transepithelial potential
-
Transcellular
- Only in the proximal straight tubule (PST)
- Cl-/base exchanger (CFEX)
-
Paracellular
-
Leaves to interstitial space (basolateral)
- Cl- channel
- K/Cl co-transporter
As Cl- is reabsorbed, H2O crosses to a greater extent.
Luminal [Cl-] in tubular fluid rises slightly as fluid moves down the PCT.
PCT
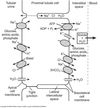
K+ Reabsorption
- Mainly through passively movement
- Paracellular
- Solvent drag in response to water movement
PCT
Ca2+ Reabsorption
~ 70% of filtered free Ca2+ reabsorbed in the PCT.
Passive and paracellular.
PCT
Phosphate Reabsorption
~ 80% of filtered Pi absorbed in PCT
Rate of transport depends on plasma [Pi] and parathyroid hormone (PTH).
Mechanism:
-
Apical absorption via 2 different Na+-phosphate cotransporters:
- NaPi IIc
- NaPi IIa
- Basolateral membrane mechanism unknown
Regulation:
- Transporters with higher affinity for HPO42- than for H2PO4-
-
pH of plasma affects reabsorption of Pi
- acidosis reduces reabsorption
-
High plasma [Pi] causes PTH release
- PTH promotes endocytosis and degradation of apical Pi transporters
- More Pi excreted
- PTH promotes endocytosis and degradation of apical Pi transporters
-
Low plasma [Pi]
- Increased transporter density on apical membrane
- Increased reabsorption of Pi

Glomerulotubular (GT) Balance
Regulation of Na+ Reabsorption
Hemodynamic changes that alter GFR modulate the rate of Na+ (and Cl-) reabsorption.
-
At normal GFR:
- Low PPC and high πPC ⇒ net fluid uptake into capillaries.
-
Increased GFR due to dec. AA resistance and inc. EA resistance:
- More fluid filtered
- Peritubular protein content increases ⇒ inc. πPC
- Hydrostatic pressure in peritubular capiilar decreases ⇒ dec. PPC
- Net effect is increased driving force for fluid movement from interstitial space ⇒ peritubular capillary
- Enhances absorption of fluid and NaCl
Neurohumoral Control
Regulation of Na+ Reabsorption
Neurohumoral stimuli:
- Affects GFR and RPF
- Via constriction/dilation of afferent and efferent arterioles
- Affect specific transporters in the kidney
- Affects Na+ reabsorption
- Hemodynamic changes have a greater effect than transporter-specific effects

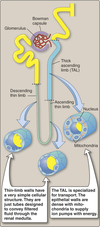
Loop of Henle
Structure
- Three sections:
- Descending thin limb (DTL)
- Ascending thin limb (ATL)
- Thick ascending limb (TAL)
- Thin limb walls:
- Very simple cellular structure
- Designed to move filtered fluid through the renal medulla
- Thick ascending limb:
- Specialized for transport
- Numerous ion pumps
- Contains many mitochondria
Loop of Henle
Permeability and Function
LoH extracts water and ions which were not reabsorbed in the proximal tubule.
70% of filtered Mg2+
25-30% of filtered Ca2+
25% of filtered Na+
15% of filtered HCO3-
~10% of filtered K+
~10% of filtered water
Each section has varied permeability and functions.
-
Descending thin limb (DTL):
- no Na+ or Cl- transport
- very water permeable
-
Ascending thin limb (ATL):
- passive Cl- transport
- passive Na+ absorption
- water impermeable
-
Thick ascending limb (TAL):
- active Na+, K+, and Cl- transport
- water impermeable
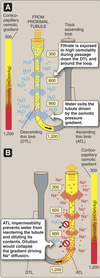
Corticomedullary Gradient
Hairpin-like structure of LoH and vasa recta coupled with the permeability properties of the various regions generates the corticomedullary gradient.
- Contained within the interstitial space.
- Gradient increases in magnitude along the length of the loop.
- Papillary tip osmolarity between 600 - 1,200 mOsm/kg depending on need to conserve water loss.
- Osmolarity of luminal fluid follows that of the corticomedullary gradient.
- Vital role in urine concentration.
Descending Thin Limb
(DTL)
- Permits water movement into interstitial space by both transcellular and paracellular pathways.
- Contains aquaporins.
-
Impermeant to ions.
- Na+ and Cl- remain in the lumen.
- Becomes progressively more concentrated as water leaves DTL.
Ascending Thin Limb
(ATL)
-
Impermeant to water
- No aquaporins
-
Permeable to Cl-
- Cl- leaves
- Na+ follows via paracellular pathway
- As Cl- and Na+ leave the lumen, tubular fluid osmolarity returns back to ~ 300 mOsm/kg.
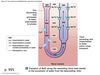
Thick Ascending Limb (TAL)
Na+, Cl-, and K+ Transport
- Basolateral Na/K-ATPase sets up an Na+ gradient.
- Na+, Cl-, and K+ ⇒ epithelial via apical Na/K/2Cl cotransporter (NKCC2) brings all three ions into epithelia
- Uses Na+ gradient as driving force
- Target for loop diuretics like furosemide
- Leads to increased urine production and loss of K+ (hypokalemia)
- Na+ ⇒ interstitial space via basolateral Na/K-ATPase
- Cl- ⇒ interstitial space via basolateral Cl- channel
- K+ can either:
⇒ interstitial space via basolateral K+ channel
⇒ lumen via apical ROMK (Renal Outer Medullary K Channel)- Creates transepithelial potential ~ +7 mV
- Drives Na+ and K+ reabsorption via paracellular pathway
- Ensures enough luminal [K+] to maintain NKCC2 function
- Creates transepithelial potential ~ +7 mV
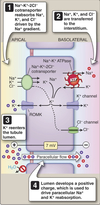
Thick Ascending Limb (TAL)
Ca2+ and Mg2+ Transport
- Ca2+ and Mg2+ reabsorption via paracellular transport
- Driven by transepithelial potential
- Inhibition of NKCC2 by loop diuretics prevents formation of transepithelial potential
- Promotes hypomagnesemia
- Ca2+ can be reabsorbed later in DCT, hypocalcemia is not seen
Thick Ascending Limb (TAL)
Bicarbonate Reabsorption
Bicarbonate reabsorbed via similar mechanism as PCT.
No luminal carbonic anhydrase.
Reaborption slower.
- TAL secretes H+ into lumen via Na+/H+ exchanger (NHE3)
- H+ converts HCO3- into H2CO3
- Spontaneous conversion of H2CO3 to CO2 and H2O
- CO2 and H2O easily enters the cell.
- Inside, carbonic anhydrase II converts CO2 back to H+ and HCO3-.
- H+ re-enters lumen via NHE3 Na+/H+ exchanger
- HCO3<strong>-</strong> moved to interstitial space by Na+/HCO3- cotransporter.
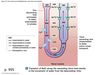
Countercurrent Multiplication
Utilizes the corticomedullary concentration gradient to reabsorb water and salt as filtrate travels through LoH.
Gradient generated by LoH and maintained by vasa recta.
~ 300 mOsm/kg @ cortical end
Up to 1,200 mOsm/kg @ medullary end
- Initial osmolarity of lumen and interstitial space is 300 mOsm/kg.
- TAL absorbs Na+ via NKCC2 transporter.
- Basolateral Na/K-ATPase transfers to interstitium.
- 200 mOsm/kg osmotic difference between lumen and interstitium generated.
-
Water leaves highly permeable DTL until equilibrium occurs with higher osmolarity in interstitium (at 400 mOsm/kg).
- ATL and TAL imperable to water and not affected.
Process occurs throughout length of TAL and DTL ⇒ single effect.
- Fluid entering DTL from proximal tubule displaces 400 mOsm/kg fluid down and around loop.
- Single effect occurs again generating higher osmolarity at the tip.
- Process continues repeatedly until osmolarity of tip 700 - 1,200 mOsm/kg based on need to conserve water.
Fluid leaving TAL into DCT more dilute than the fluid entering LoH.

Nephron Coupling
Urea
Characteristics
- Urea comprises ~ half of solutes in deep renal medullary interstitium.
- Diffuses poorly due to small polar nature.
- Urea transporters faciliate movement across membranes.
- Unilateral vs bilateral
- Pool of urea in the deep renal medullary interstitium augments the corticomedullary gradient.
- Osmolarity would be 600 instead of 1,200 mOsm/kg without urea.
Urea
Transport
-
Collecting ducts secretes urea into interstitium of inner medulla via unilateral UT-As.
- Apical UT-A1 requires ADH/AVP to operate
- Basolateral UT constiutive
- Urea enters tip of LoH via unilateral UT-Bs.
- Urea carried through distal segment and returns to collecting duct.
-
Urea recycling results in accumulation of urea in interstitium at tip of LoH.
- Contributes to corticomedullary gradient.
-
Vasa recta contains bilateral UT’s.
- Urea in vasa recta equilibrates with interstitial urea.
- Helps maintain osmotic gradient.
- Some can be returned to systemic circulation.
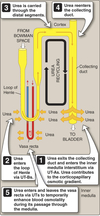
Urea Excretion
Control
Amount of urea excreted vs recycled depends on the body’s water conservation needed.
-
Decreased water intake:
- ADH/AVP released from posterior pituitary.
- ADH ⇒ GPCR ⇒ cAMP ⇒ PKA
- Activates apical UT-A1 of collecting duct
- Urea recycled, excretion rates minimal.
- ADH/AVP released from posterior pituitary.
-
Increased water intake:
- ADH/AVP release suppressed
- UT-A1 in collecting ducts operate at low levels
- Urea excreted in the urine
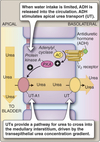
Distal Nephron
Properties
- Consists of the:
- Distal convoluted tubule (DCT)
- Connecting tubule (CNT)
- Cortical collecting ducts (CCD)
- Most of the Na+ and water has been reabsorbed from the filtrate at this point.
- Actual amount absorbed in the distal nephron much lower than PCT or LoH
- ~ 10% filtered Na+
- ~ 20% filtered water
- Actual amount absorbed in the distal nephron much lower than PCT or LoH
- Regulated by hormones associated with Na+ and water homeostasis
- Becomes an important point for modifying water and electrolyte transport in response to changing conditions

Distal Convoluted Tubule
Properties
- Impermeant to water even in the presence of ADH/AVP
- Absorbs ~ 5% of filtered Na+
- Movement of salts without water causes tubular fluid in DCT to be hypo-osmolar to interstitium
Distal Convoluted Tubule
Na+ and Cl- Reabsorption
-
Na+ and Cl- from lumen into cell via apical Na/Cl co-transporter (NCC)
- Site of action for tiazide diuretics such as hydrochlorothiazide
- Na+ leaves via basolateral Na/K-ATPase
- Cl- leaves via basolateral Cl- channel

Distal Convoluted Tubule
Ca2+ Reabsorption
Together the DCT and CNT reabsorbs ~ 8% of filtered Ca2+.
- Ca2+ crosses the apical membrane via TRPV5 Ca2+ channel.
- Driving force is the electrochemical gradient for Ca2+
- Ca2+ binds to calbindin in the cytosol
- Maintains the low cytosolic [Ca2+] required by cells.
- Ca2+/calbindin complex translocated to the basolateral membrne.
- Ca2+ crosses basolateral membrane via either:
- Ca2+-ATPase
- Na/Ca exchanger
Transport of Ca2+ regulated by PTH.
- When plasma [Ca2+] low, PTH released from parathyroid.
- PTH acts via a GPCR through both adenylyl cyclase and phospholipase C.
- Leads to an increase in:
- TRPV5 open probability
- Activity of both basolateral Ca2+-ATPase and Na/Ca exchanger
- PTH effects potentiated by Vit D (calcitriol)
- Vit D increases synthesis of most (if not all) proteins involved in Ca2+ transport.
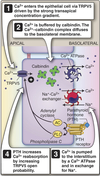
Connecting Tubule
(CNT)
Properties and Transport
CNT has transport systems similar to both DCT & CCD.
- Na+ and Cl- enter apical membrane via NCC.
- Na+ also enters via apical aldosterone-sensitive epithelial Na+ channel (ENaC)
- Na+ leaves via basolateral Na/K-ATPase
- Cl- leaves via basolateral Cl- channel
-
Ca2+ handled by similar PTH-sensitive pathway seen in DCT
- Apical TRPV5 Ca2+ channel
- Basolateral Ca-ATPase and Na/Ca exchanger
- CNT is permeable to water in the presence of ADH/AVP.
- Increases the levels of aquaporin 2 (AQP2)
Cortical Collecting Ducts
Cell Types
Three functionally-distinct cell types in the CCD:
-
Principal cells
- Absorbs Na+ via ENaC
- Secretes K+ into lumen
- Absorbs water in the presence of ADH/AVP
-
𝛼-intercalated cells
- Secretes H+
- Generates new HCO3-
- Reabsorbs K+ when plasma [K+] is very low
-
𝛽-intercalated cells
- Secretes HCO3-
- Reabsorbs K+ when plasma [K+] is very low
Cortical Collecting Ducts
Na+ and Cl- Transport
- Basolateral Na/K-ATPase sets up Na+ gradient
-
Na+ reabsorbed by apical ENaC
- Utilizes Na+ gradient as driving force
- Blocked by amiloride
- K+-sparing diuretic
- Na+ ⇒ insterstitial space via Na/K-ATPase
-
Transcellular Na+ movement generates a large transepithelial potential of ~ -40 mV in the lumen
- Much greater than other sites of the nephron
- Transepithelial potential provides strong driving force for paracellular Cl- movement lumen ⇒ interstitium.
-
𝛽-intercalated cells reabsorb Cl- via apical Cl/HCO3- exchanger (Pendrin).
- 𝛼-intercalated cells do not participate in Cl- transport
- Cl- ⇒ interstitium via Cl- channel
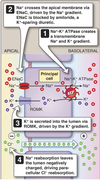
Cortical Collecting Ducts
K+ Transport
-
K+ is secreted by principal cells.
- K+ moves into cell from interstitium by Na/K-ATPase
- Enter lumen via ROMK channel
- Driving force is K+ concentration gradient and negative potential within lumen.
- If Na+ reabsorption blocked in TAL by loop diurectics or DCT by thiazide diuretics
- more Na+ appears at principal cells
- more Na+ reabsorbed here
- lumen more electronegative facilitating K+ loss into lumen ⇒ explains hypokalemia associated with both classes of diuretics
-
𝛼-intercalated cells can reabsorb K+ via apical H/K-ATPase by exchange with H+
- Normally minimal
- Becomes important when plasma [K+] very low
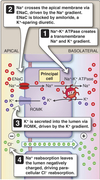
Cortical Collecting Ducts
Bicarb and Acid Transport
Handled in the distal nephron by intercalated cells.
-
𝛼-intercalated cells are the major contributor.
- H+ secreted into lumen via apical H+-ATPase and H/K-ATPase
- HCO3- ⇒ interstitial space via Cl/HCO3- exchanger (Pendrin class)
- Able to synthesize HCO3- via carbonic anhydrase
-
𝛽-intercalated cells
- HCO3- secreted into lumen via apical Cl/HCO3- exchanger (Pendrin, different than ones found in 𝛼 cells)
- H+⇒ interstitial space via basolatral H+-ATPase and H/K-ATPase
The opposing ability of 𝛼 and 𝛽 cells to secrete titratable acid important in acid/base balance.

Cortical Collecting Ducts
Regulation of Na+ Recovery
Aldosterone regulates Na+ recovery by principal cells.
- Aldosterone produced by RAAS system in response to:
- Decreased blood pressure
- Decreased renal blood flow
- Binds to basolateral mineralocorticoid receptor (MR)
- Ligand/receptor complex translocated to nucleus
- Expression of genes for a number of proteins upregulated including:
- ENaC
- ROMK
- Na/K-ATPase
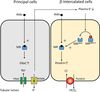
Cortical Collecting Ducts
Regulation of Water Recovery
Water reabsorption by principal cells of CCD regulated by ADH/AVP.
-
Aquaporin 2 (AQP2) constantly expressed in principal cells
- Sequestered in vesicles in the absence of ADH/AVP
-
Basolateral membrane always contains AQP3 and AQP4
- However, no transcellular water movement without apical AQP2
-
ADH/AVP released from posterior pituitary in response to:
- Increase in plasma osmolarity
- Decrease in circulating blood volume
- Acts via V2 vasopressin receptors
- Causes AQP2 translocation and insertion into apical membrane
- Completes the transcellular pathway for water movement.
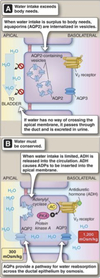
Nephron Transport
Summary

ECF
Osmolarity
ECF osmolarity determined by [Na+]ECF
Approximate the ECF osmolarity using ECF solute content and ECF solute volume:
ECFosmolarity = ECFsolute content / ECFvolume
ECF
Volume
Almost all of the ECF solute content is Na+ and it’s associated anions (Cl-, HCO3-).
Estimate ECF volume as:
ECFvolume = (2 x ECFNa+ content) / ECFosmolarity
ECF osmolarity is tightly controlled.
ECF volume varies with Na+ content.
Alterations in Na+ reabsorption and/or excretion used to maintain a more or less constant ECF volume in the face of varying conditions.
Effective Circulating Volume
The volume on the arterial side of the circulation that provides sufficient tissue perfusion for ongoing metabolic and functional needs.
Systems which maintain a constant ECF via alterations in Na+ reabsorption/excretion monitor the effective circulating volume rather than ECF itself.
General rule:
Increased effective circulating volume ⇒ ↓Na+ reabsorption and ↑Na+ excretion.
Decreased effective circulating volume ⇒ ↑Na+ reabsorption and ↓Na+excretion.
Baroreceptors
Blood volume affects vascular pressure.
Baroreceptors used to sense effective circulating volume.
-
Arterial baroreceptors
- located in carotid sinus and aortic arch
- high-pressure baroreceptors
- mediate the classic cardiovascular baroreceptor reflex
- Increased BP ⇒ increased firing ⇒ inhibition of SNS gas & activation of PNS break
-
Cardiopulmonary baroreceptors
- located in atria and pulmonary arteries
- low-pressure baroreceptors
- essentially sense blood volume
-
Intrarenal baroreceptors
- located in granular cells of the afferent arteriole
- play a role in control of renin release by granular cells

Regulation of Na+ Reabsorption
by
GT Balance
Ensures constant filtration by the proximal tubule in the face of changing GFR.
Proximal tubule reabsorbs essentially a constant fraction of the Na+ presented to it to ensure that Na+ is not inappropriately reabsorbed with changes in GFR.
Controlled via the effects of GFR changes on the Starling forces of the peritubular capillaries which governs fluid reabsorption.
Increased GFR leads to increased reabsorption by the peritubular network ensuring a relatively constant Na+ reabsorption rate.
-
At normal GFR:
- Low PPC and high πPC ⇒ net fluid uptake into capillaries.
-
Increased GFR:
- More fluid filtered
- Peritubular protein content increases ⇒ increased πPC
- Hydrostatic pressure in peritubular capillary decreases ⇒ dec. PPC
- Net effect is increased driving force for fluid movement from interstitial space ⇒ peritubular capillary
- Enhances absorption of fluid and NaCl
Sympathetic Control
Na+ Reabsorption
-
SNS alters renal blood flow and GFR
- Acts via 𝛼1-adrenergic receptors
- Mediates vasoconstriction of afferent and efferent arterioles
- Na+ excretion tends to move in the same direction as GFR
- If GFR falls, tubules absorb proportionally more Na+ so Na+ excretion falls
- However, due to significant amount of autoregulation, GFR changes are minimal under normal conditions.
-
SNS upregulates Na+ transport systems in the proximal tubule via 𝛼-adrenergic stimulation
- Increased activity of apical NHE3 Na+/H+ exchanger
- Increased activity of basolateral Na+/K+-ATPase
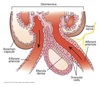
Juxtaglomerular Apparatus
(JGA)
Important role in the RAAS system:
Consists of:
-
Macula densa
- epithelial cells in the distal end of the TAL of LoH
- releases ATP in response to increased tubule NaCl delivery
- ATP ⇒ Adenosine ⇒ A1 purine receptors on afferent arterioles and A2 purine receptors on efferent arterioles
- Results in vasoconstriction of afferent arterioles and vasodilation of efferent arterioles ⇒ reduces RBF and GFR
-
Granular cells
- located in the afferent arterioles mostly
- Renin production and release
-
Extraglomerular mesangial cells
- serves as a communication conduit between the macula densa and granular cells
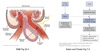
Renin-Angiotensin-Aldosterone System
(RAAS)
Overview
Major role in controlling Na+ reabsorption/excretion and water balance.
- Renin released from granular cells of JGA
- Rate-liming step in the RAAS system
- Site of regulation
- Renin cleaves circulating angiotensinogen ⇒ angiotensin I
- Angiotensin I processed by ACE in the lungs ⇒ angiotensin II
- Ang II is the biologically-active form of angiotensin
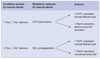
Stimuli for Renin Release
-
Sympathetic nervous system
- Renal nerve innervates afferent arterioles
- Activation of 𝛽1-adrenergic receptors on granular cells leads to renin release
- Provides a level of control driven by changes in systemic vascular pressure
- Mediated by high-pressure baroreceptors
- Drop in systemic BP elicits renin release
- Increase in systemic BP inhibits renin release
-
Afferent artiole pressure
- Granular cells act as intrarenal baroreceptors
- Pressure drop in the afferent arteriole ⇒ increased renin release
-
Macula densa
- Senses both tubular flow and tubular NaCl content
- When flow/NaCl content too high ⇒ ATP released
- Reduces GFR
- Inhibits renin secretion
- When flow/NaCl content too low ⇒ PGE2, NO, and other mediators released by extraglomerular mesangial cells
- Increases GFR
- Increases renin secretion
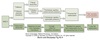
RAAS Effects
Ang II has multiple effects that alters the ability of the kidney to absorb both water and Na+:
-
Stimulation of aldosterone production by adrenal cortex
- Aldosterone increases activity of apical ENaC and basolateral Na+/K+-ATPase in the collecting duct
- Results in increased Na+ reabsorption
-
Increases NHE3 and Na/K-ATPase activity in the proximal tubule
- Results in increased Na+ reabsorption
-
Stimulates thirst and secretion of AVP/ADH
- Both increase water reabsorption
-
Preferentially vasoconstricts the efferent arterioles
- Increases GFR
- Reduces peritubular hydrostatic pressure
- Increases peritubular oncotic pressure
- Net effect:
- increased Na+ and water reabsorption by the PCT
- increased fluid movement into the peritubular capillaries
- Reduces blood flow to the vasa recta
- stabilizes and deepens the corticomedullary gradient
- enhances passive Na+ reabsorption by the ATL
Arginine Vasopressin
(AVP/ADH)
Major effect is to maintain water balance.
Increases NKCC2 activity in TAL of LoH.
Increases ENaC activity of principal cells in collecting ducts.
Promotes Na+ reuptake.
Atrial Natriuretic Peptide
(ANP)
ANP released when blood volumes get too high.
Antagonizes actions of RAAS.
Contribution of ANP to overall Na+ and water balance is minimal under normal conditions.
- Vasodilates afferent arterioles ⇒ increasing GFR
- Inhibits Renin and AVP/ADH release
- Net result is increased fluid flow but decreased reabsorption
- Leads to diuresis and natriuresis
Dopamine
Synthesized in the proximal tubule.
Increases in Na+ intake ⇒ increased dopamine production.
Exact mechanism unclear.
Dopamine has several effects:
- Dilates afferent arterioles ⇒ increases RBF and GFR
- Causes removal of NHE3 and Na/K-ATPase from proximal tubule ⇒ decreases transcellular absorption.
- Reduces expression of Ang II receptors ⇒ prevents effects of Ang II specifically upregulation of NHE3 and Na/K-ATPase in the PT
Sodium Regulation
Summary

Potassium Balance
[K+]ECF = 3.5 - 4.5 mEq/L
[K+]ICF = ~ 140 mEq/L
- Concentration gradient is set up by the Na/K-ATPase.
- Responsible for the resting membrane potential of cells.
- Small alterations in [K+]ECF can have significant affects on membrane potential and ability to fire action potentials.
- Both hypokalemia and hyperkalemia can cause cardiac arrhythmias.
-
Two processes considered in K+ balance:
- Distribution of K+ between ECF and ICF
- Total amount of K+ in the body
Skeletal Muscle
Potassium Content
Skeletal muscle represents 2/3 of total cell mass and thus 2/3 of total K+
Abnormal leakage of K+ from muscle can result in dangerously high levels of [K+]ECF
Can occur with crush injury or rhabdomyolysis.
Potassium
ECF/ICF Distribution
[K+]ECF = 3.5 - 4.5 mEq/L and [K+]ICF = ~ 140 mEq/L
Majority of K+ is within the ICF.
Movement of small amounts of K+ into or out of the ICF can have significant affects.
Two most important factors influencing distribution of K+ are activity of Na/K-ATPase and serum [K+].
Example: 60 kg female with ~ 20 L of ICF and ~ 10 L of ECF
[K+]ICF @ 140 mEq/L ⇒ 2,800 mEq
[K+]ECF @ 4 mEq/L ⇒ 40 mEq
If 2% of total ICF K+ content (56 mEq) moved into the ECF, this would increase [K+]ECF to 9.6 mEq/L ⇒ lethal level
K+ Movement
Into Cells
Na/K-ATPase pumps K+ into the ICF against its concentration gradient.
Na/K-ATPase activity increased by several factors:
Promotes movement of K+ into the ICF
- Increased plasma [K+]
- Insulin (+ glucose sometimes used as a treatment for hyperkalemia)
- Epinephrine via 𝛽2 adrenergic stimulation
- Thyroid hormone
- Aldosterone through principal cells in collecting ducts
- Alkalosis
Na/K-ATPase activity impaired by several factors:
Inhibits movement of K+ into the ICF
- Cardiac glycosides (i.e. digoxin)
- Chronic renal failure
- Congestive heart failure
- Acidosis
K+ Movement
Out of Cells
Several factors increase K+ movement out of cells and into the ECF:
-
Exercise
- K+ leaves myocytes during membrane depolarization
- transient event as Na/K-ATPase moves K+ back into cells under normal conditions
-
Increased serum osmolarity
- cells shrink increasing intracellular [K+]
- promotes diffusion of K+ out of the cell
- can be seen with uncontrolled diabetes
-
Acidosis
- increased extracellular [H+] promotes H+ entry into cells
- results in subsequent K+ loss
- acts as a virtual H+/K+ exchanger
- no such transporter exists
- mechanism may involve the stimulatory effects of acidosis on Na/K-ATPase
K+ Shift
Summary
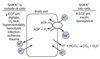
Total Body K+ Content
Movement of K+ between ECF/ICF provides moment-to-moment control of plasma [K+]
Total body K+ content determined by the kidneys
Normal daily intake of K+ is 50-150 mEq/day.
K+ readily absorbed by the GI system in an unregulated fashion
Equal amount must be excreted to remain in K+ balance
90% excreted via the kidneys, 10% excreted via the feces
K+ balance maintained by adjusting renal reabsorption and excretion of K
Renal Handling of K+
- Filtered load of Na+ is 30-40x greater than K+
-
K+ is both absorbed and secreted
- Na+ is always reabsorbed
- Regulation of K+ balance done via regulation of secretion
- Renal handing of Na+ has a greater effect of handling of K+ than K+ on Na+
- Major point of K+ control
- K+ is freely filtered into Bowman’s space
- ~ 90% of K+ is reabsorbed by the PCT and TAL of LoH
-
Collecting tubules and collecting ducts can reabsorb and/or secrete K+
- ~90% of filtered K+ has been absorbed by the time filtrate reaches the distal nephron regardless of intake
- Location of fine-tuning of K+ balance to match K+ intake
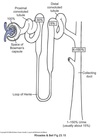
PCT
Potassium Handling
PCT reabsorbs ~ 70% of filtered K+ mostly via paracellular route.
Driven by two mechanisms:
-
Transcellular Na+ movement sets up an osmotic gradient
- Drives water movement across PCT via paracellular route
- Creates solvent drag with sweeps K+ along with the water
- Greater Na+ reabsorption ⇒ greater H20 movement ⇒ greater K+ movement
-
Transepithelial potential varies along PCT length becoming slightly positive at the distal end of PCT
- Facilitates K+ movement via paracellular route in this region
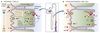
TAL
Potassium Handling
TAL reabsorbs ~ 10% of filtered K+ via paracellular and transcellular mechanisms.
- Lumen-positive transepithelial potential drives K+ movement via paracellular route.
-
Bulk of K+ reabsorption via transcellular route by NKCC2 cotransporter
- Moves Na+, K+, and Cl- across the lumen
- K+ crosses basolateral membrane via:
- K+ channel
- K/Cl cotransporter
-
Luminal ROMK channel recycles some K+ back into the lumen
- Moves down its concentration gradient
- Ensures sufficient luminal K+ to support NKCC2 function
- If this did not happen, Na+ and Cl- reabsorption would be limited by [K+] in the filtrate
Bartter Syndrome
Electrolyte disorder characterized by reduced reabsorption of Na+, K+, and Cl-.
One form caused by a defective ROMK channel.
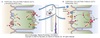
Principal Cells
Potassium Handling
Responsible for secretion of K+ in the collecting tubules.
-
Basolateral Na/K-ATPase moves K+ from interstitium into cell and Na+ into interstitial space
- Creates driving force for K+ movement from cell to lumen
- Creates driving force for Na+ movement from lumen into cell
- K+ moves down its electrochemical gradient from cell ⇒ lumen via apical ROMK channel
- Enhanced by negative transepithelial potential
-
K+ channels present in both apical and basolateral membranes
- More K+ channels on apical membrane
- Negative transepithelial potential favors K+movement from cell into lumen
- Fraction of K+ that does cross basolateral membrane moved back into the cell by Na/K-ATPase
-
Increased entry of Na+ via ENac stimulates Na/K-ATPase
- Increases driving force for K+ secretion
- Explains hypokalemia seen with use of loop and thiazide diuretics
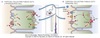
𝛼-Intercalated Cells
Potassium Handling
Responsible for reabsorption of K+ in the collecting tubules.
-
Apical H/K-ATPase pumps K+ into cell from lumen and H+ into the lumen
- Important in acid-base regulation
- K+ moves from cell into interstitium via basolateral K+ channels
Mechanism
of
K+ Secretion Regulation
The key regulated step of K+ balance is K+ secretion by principal cells.
- Three transport processes determine the amount of K+ secretion:
- K+ influx from interstitial space by Na/K-ATPase
- K+ efflux into lumen
- K+ efflux into insterstitial space (recycling)
Majority of control centered on the activity of K+ channels
Two types of K+ channels in the apical membrane of principal cells:
ROMK and BK
Each has a different role and regulated by different mechanism.
-
At low dietary K+ ⇒ low K+ filtered load
-
Both ROMK and BK channels inactive
- ROMK sequestered in intracellular vesicles
- BK channels closed
- Low level of K+ secretion
-
Both ROMK and BK channels inactive
-
At normal K+ loads
- ROMK channels moved to apical membrane
- BK channels are still closed
- Moderate level of K+ secretion
-
At high K+ loads
- Both ROMK and BK present and active in apical membrane
- High level of K+ excretion

Plasma [K+]
Effects on K+ Secretion
↑ plasma [K+] ⇒ ↑ K+ secretion
- filtered load of K+ proportional to plasma concentration [K+]
- interstitial space [K+] essentially the same as plasma [K+]
- Na/K-ATPase pumping rate dependent on extracellular [K+]
- important but not dominant controlling factor
Dietary K+ Intake
Effects on K+ Secretion
↑ Dietary [K+] ⇒ ↑ K+ secretion
- renal secretion must match dietary intake
- kidney adjusts K+ excretion to match intake
- mechanism unclear, may involve GI peptide hormones
Aldosterone
Effects on K+ Secretion
Aldosterone synthesis and secretion also stimulated by increased plasma [K+] independent of Ang II mechanism.
Aldosterone:
- Stimulates ENaC activity ⇒ increased Na+ entry
- High Na+ entry stimulates Na/K-ATPase activity ⇒ increased K+ entry
- Increased intracellular [K+] stimulates ROMK activity
- Facilitates K+ secretion by ROMK
Spironolactone and eplerenone block the aldosterone receptor ⇒ Reduces K+ secretion.
Hypoaldosteronism ⇒ hyperkalemia
Hyperaldosteronism ⇒ hypokalemia
Na+ Delivery to Principal Cells
Effects on K+ Secretion
Amount of Na+ delivery to prinical cells is a major regulator of K+ secretion.
- High [Na+] at the distal nephron increases Na+ entry via ENaC
- Leads to high Na/K-ATPase activity increasing K+ entry from interstitium
- Elevated intracellular K+ facilitates secretion via ROMK
- Explains hypokalemia seen with loop and thiazide diuretics
- Amiloride or triamterene blocks ENaC ⇒ reduces K+ secretion
Tubular Flow Rate
Effects on K+ Secretion
High flow rates ⇒ decreased luminal [K+] at principal cells.
Facilitates K+ secretion.
High flow rates ⇒ increased Na+ reabsorption.
Facilitates K+ secretion.
AVP/ADH
Effects on K+ Secretion
AVP/ADH has minor effects on K+ secretion.
- Increases ENaC conductance ⇒ provides larger driving force for K+ secretion.
- Increases apical K+ permeability
- However, reduces urine flow ⇒ reduce K+ secretion
Most likely the effects of AVP/ADH cancel themselves out.
Potassium Secretion Effectors
Summary

Primary Acid-Base Disorders
There are two primary acid-base disorders:
Acidosis ⇒ gain H+ or lose HCO3-
Alkalosis ⇒ lose H+ or gain HCO3-
There are two ways of developing a primary acid-base disorder:
Respiratory and Metabolic
There are two states of responses:
Compensated and Uncompensated

Davenport Diagram
Describes the relationship between plasma pH and [HCO3-] at a various PCO2 values.
PCO2 Isopleths: shows how pH depends on [HCO3-] at a constant PCO2.
Normal buffer line: shows the total buffering capacity of the system (HCO3- and NBB).
Intersection of the two curves represents when both types of buffers are in equilibrium.
We must exist at a point of intersection.

Acid-Base Homeostasis
Mechanisms
-
Buffer systems
- chemical buffers found in ECF and ICF
- minimalizes changes in pH until compensation can occur
- does not remove the excess acid or base from the body
- works immediately as pH changes
-
Lungs
- respiratory control centers in the brainstem adjust ventilation rate
- increases or decreases CO2 transfer to the atmosphere
- changes can take place very quickly
- compensatory response occurs within minutes
-
Kidneys
- can adjust the amount of H+ and HCO3- secreted and excreted
- involves upregulation of various enzymes and transporters
- full renal compensation may take up to several days
Successful compensation for a primary acid-base distrubance returns the pH close to baseline but normalization may not occur until underlying cause is removed.

Buffer Pair
A weak acid and it’s conjugate base.
Relative concentrations determined by the dissociation constant.
Changes in pH will affect the acid/conjugate base ratio and vice versa.
Explicit relationship given by the Henderson-Hasselbach equation:
pH = pKeq + log ( [A-]/[HA] )
Physiological Buffers
Two broad categories of buffer systems:
-
Bicarbonate buffer system (CO2/HCO3-)
- Most important buffer pair in the ECF
-
Non-bicarbonate buffers in both ECF and ICF
- Blood: proteins, H2PO4-/HPO42-
- Interstitial fluid: H2PO4-/HPO42-
- Intracellular: proteins, H2PO4-/HPO42-, phosphate constituents of organic compoounds (e.g. ATP)
Bicarbonate Buffer System
Extremely important in acid-base physiology due to:
-
Abundance of components
- [HCO3-]ECF ~ 24 mM
- metabolism provides unlimited CO2
-
Open system
- components can be added or removed from the body at controlled rates
- Control by the lungs and kidney
Buffering in the system limited by the amount of available HCO3-
Carbonic anhydrase catalyzes formation of carbonic acid.
Using Henry’s Law for [CO2]dissolved
Henderson-Hasselbach equation for the system
pH = 6.1 + log [HCO3-] / 0.03 PCO2
pH can be controlled by alternating PCO2 or [HCO3-].
Non-Bicarbonate Buffers
(NBBs)
CO2 + H20 ⇔ HCO3- + H+
+
NBB - ⇔ NBBH
Bicarb and plasma NBBs “share” added H+.
Pulls reaction to the right.
Renews available bicarb enhancing buffering capacity.
Renal
Acid-Base Handling
Kidneys remove H+ if there is excess acid.
Most H+ secreted as either:
NH4+
Combined with urinary buffers such as phosphate ⇒ titratable acid
Kidneys remove HCO3- if there is excess base.
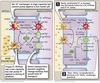
Urinary Acidification
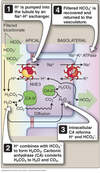
Several portions of the nephron secrete H+ into the lumen.
-
PCT (left panel)
- via NHE3 Na+/H+ exchanger
- majority of acid secreted
-
TAL
- also via the NHE3 Na+/H+ exchanger
-
𝛼-intercalated cells of the collecting duct (right panel)
- excrete H+ via an H/K ATPase or H+-ATPase
HCO3- Reabsorption
Tubules reabsorb essentially all filtered HCO3- mainly in the PCT.
Involves carbonic anhydrase.
H+ released by intracellular H2CO3 breakdown combines with filtered HCO3- and cycles around.
Does not result in H+ secretion.
No net creation of HCO3- ⇒ only a reclamation process.
Generation of New HCO3-
H+ and HCO3- produced from CO2 by carbonic anhydrase.
H+ combines with filtered HPO42- ⇒ excreted
HCO3<strong>-</strong> formed enters blood via basolateral Na/HCO3- cotransporter
For every H+ excreted as a titratable acid or ammonia, a new HCO3- is added to the blood.
Loss of H+ in the urine is equivalent to adding new HCO3- to the blood.
Loss of HCO3- from the body equivalent to adding H+ to the blood.
Supply of phosphate and other NBBs limited.
Ammonia excretion used to excrete large amounts of acid.
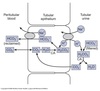
Ammonia Excretion
Majority of ammonia synthesized in the proximal tubule from glutamine.
Ammonia can enter urine:
As lipid-soluble NH3
As NH4<strong>+</strong> via NHE3 where NH4+ can substitute for H+
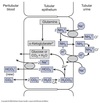
Acid-Base Balance
Clinical Evaluation
Titratable acid measured ex vivo via titration of urine with NaOH until pH 7.4
Represents the amount of H+ excreted combined with urinary buffers.
Ammonia determined via a seperate method.
Net Acid Excretion
During stable acid-base balance:
Net acid secretion = net addition of H+ by metabolic processes.
Net acid excretion = net HCO3- production.
Excretion of HCO3- represents a loss of base and must be subtracted.
Net acid excretion (mEq/day) = urinary titratable acid excreted + urinary NH4+ excreted - urinary HCO3- excreted.
NAE with normal plasma pH of 7.4 ~ 60-80 mEq/day.
Much higher with acidosis due to increased NH4+ excretion.
Much lower or negative with alkalosis due to increased HCO3- excretion.

Primary Respiratory Acidosis
↑ PCO2 ⇒ ↓ [HCO3-]/PCO2 ratio ⇒ ↓ pH
Caused by decreased alveolar ventilation resulting in CO2 retention.
(airway obstruction, chest trauma, neuromuscular disease affecting breathing, inadequte mechanical ventilation)
Respiratory failure “closes” the bicarbonate buffer system because CO2 cannot leave.
Reduces effectiveness of bicarbonate as a buffer.
Uncompensated respiratory acidosis with PCO2 40 mmHg → 80 mmHg results in a pH ~ 7.2 and [HCO3-] ~ 28 mM.
Compensation for prolonged respiratory acidosis through increased renal H+ secretion and HCO3- reabsorption.
Requires 12-24 hours to become evident and ~5 days for max effect.

Primary Respiratory Alkalosis
↑ [HCO3-]/PCO2 ratio ⇒ ↑pH
Caused by increased elimination of CO2 due to hyperventilation.
(general respiratory stimuli e.g. anxiety or fever, mechanical or voluntary hyperventilation, peripheral respiratory stimuli e.g. altitude, pneumonia, CHF)
Uncompensated respiratory alkalosis with PCO2 40 mmHg → 20 mmHg results in pH ~ 7.6 and [HCO3-] ~ 20 mM.
Renal compensation via decreased H+ secretion with subsequent drop in HCO3- synthesis and increased excretion of filtered HCO3-.
Brings [HCO3-]/PCO2 ratio back towards normal.
Evident within 1-2 hours and max effect in 24-48 hours.

Anion Gap
Anion gap = [Na+] - ( [Cl-] + [HCO3-] )
Cations must equal anions.
[Cl-] + [HCO3-] ≠ [Na+]
Due to unmeasured anions (proteins and organic acids)
Normal anion gap ~ 9-16.
Greater than normal anion gap means an unmeasured anion is elevated.

Primary Metabolic Acidosis
Decreased [HCO3-]/PCO2 ratio due to loss of HCO3- or excessive production of H+.
-
Excessive HCO3- loss
- Due to GI or renal causes
- diarrhea ⇒ reduces ability of GI tract to reabsorb HCO3-
- failure to secrete enough H+ ⇒ incomplete reabsorption or insufficient generation of HCO3-
- Cl- levels increase as HCO3- levels fall
- Anion gap is normal
- Due to GI or renal causes
-
Increased H+ generation
- Excessive production of organic acids such as lactate or ketoacids
- Starvation or uncontrolled diabetes
- Ingestion of drugs or poisons
- Ethanol, ethylene glycol, or aspirin
- Cl- levels do not rise so [Cl-] + [HCO3-] is not constant
- Anion gap is elevated
- Excessive production of organic acids such as lactate or ketoacids
Uncompensated metabolic acidosis moves down to lower buffer line with pH ~ 7.2 and [HCO3-] ~ 16 mM.
Initial respiratory compensation ↓ PCO2 by ↑ minute ventilation ⇒ ↑ [HCO3-]/PCO2 ratio.
Takes 15-30 minutes, time for ∆ [H+] in CSF to affect respiration.
If acidosis persists, renal compensation ↑ H+ secretion ⇒ ↑reabsorption/synthesis HCO3- ⇒ ↑ [HCO3-]/PCO2 ratio.

Primary Metabolic Alkalosis
↑ [HCO3-]/PCO2 ratio ⇒ ↑pH
-
Due to bicarbonate gain
- administration of bicarb or precursor at rate that exceeds elimination
-
Due to net H+ loss
- vomiting and loss of HCl from GI
- loop diuretics or thiazide diuretics causing ↑ renal H+ secretion
Uncompensated metabolic alkalosis moves buffer line up with pH ~ 7.5 and [HCO3-] ~ 30 mM.
Respiratory compensation ↑PCO2 by ↓ ventilation ⇒ ↓ [HCO3-]/PCO2 ratio.
Limited by respiratory drive due to hypoxemia and hypercapnia.

Acid-Base Compensation
Summary
Overall goal of compensation to return [HCO3-]/PCO2 ratio back to normal (~20).

Winter’s Formula
Acidosis
PCO2 = {(1.5 x [HCO3-]) + 8} ± 2
Used to predict the degree of respiratory compensation in metabolic acidosis.
Measured PCO2 within calculated range ⇒ adequate respiratory compensation has taken place.
Measured PCO2 outside of range ⇒ concomitant respiratory acid-base disorder also present.
PCO2 > calculated value ⇒ respiratory acidosis.
PCO2 < calculated value ⇒ respiratory alkalosis.
Rule of thumb:
If there is adequate respiratory compensation, PCO2 ≈ last 2 digits of pH.
Winter’s Formula
Alkalosis
PCO2 = (0.7 x [HCO3-]) + 20
Designed to analyze respiratory compensation of metabolic alkalosis.
Infrequently used.
Lower Urinary Tract (LUT)
Functions
Continence ⇒ urine storage.
Bladder acts as a reservoir.
Micturition ⇒ periodic elimination of urine.
Bladder neck, urethra, and urethral sphincter acts as an outlet.
Lower Urinary Tract
Innervation
Peripheral nervous system regulation of LUT coordinated by
Pontine Micturition Center and Pontine Storage Center
Both located in the rostal pons.
-
Parasympathetic innervation of LUT via pelvic splanchnic nerves
- originates in the sacral spinal cord
-
Sympathetic innervation of the LUT via hypogastric nerves
- originates in the thoracolumbar spinal cord
-
Somatic innervation of the external urethral sphincter via pudendal nerves
- originates in the sacral spinal cord

Bladder
Functional Anatomy
- Bladder wall is composed of detrusor smooth muscle
-
Innermost lining of the bladder is the urothelium
- important protective barrier
- prevents exposure of bladder musculature and CT to urine
- Bladder has two functional regions:
-
Body (fundus)
- top 2/3 of bladder
- storage chamber
- pump for urine expulsion
- densely innervated by parasympathetic nerves
- sparsely innervated by sympathetic nerves
-
Base
- lower 1/3 of bladder
- functions as a funnel
- comprised of two subsections:
-
Trigone
- posterior, lower region
- bordered by the ureter entrance points (ureterovesical junction) and the urethral orifice
-
Neck
- funnel-shaped extension of the bladder
- connects with the urethra
- wall composed of an outer longitudinal and inner circular layer of smooth muscle
- richly innervated by sympathetic nerves
- posseses mainly 𝛼1 receptors
-
Trigone
-
Body (fundus)
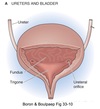
Bladder ⇒ Ureter Backflow
Prevention Mechanisms
- Flap-like valve separates the ureters from the bladder
-
Anatomical arrangement of the ureters entrance into the bladder
- ureters pass obliquely through bladder wall
- bladder smooth muscle contraction compresses the ureters
- prevents retrograde flow during micturition
- peristaltic contraction of ureters during urine movement creates a pressure wave which opens entrance
Vesicoureteral Reflux
- Abormality often seen in children
- Causes:
- congeital abnormality
- short intravesical ureter
- reflux may resolve with age
- urinary tract infections
- if ureters become inflamed
- may lead to pyelonephritis and renal scarring
Female Urethra
Functional Anatomy
- only 3-4 cm long
-
Smooth muscle throughout entire length
-
inner longitudinal
- important for urethral shortening and funneling during micturation
-
outer circular
- aid in sphincteric mechanism
-
inner longitudinal
- Mucus-secreting glands throughout
Male Urethra
Functional Anatomy
- Classified into 4 regions:
- Preprostatic urethra
- Prostatic urethra
- Membranous urethra
- Penile urethra
- 18-20 cm long
-
Smooth muscle throughout entire length
-
inner longitudinal
- important for urethral shortening and funneling during micturation
-
outer circular
- aid in sphincteric mechanism
-
inner longitudinal
- Thickened ring of smooth muscle within the preprostatic urethra ⇒ lissosphincter
- Mucus-secreting glands throughout
Internal Urethral Sphincter
(IUS)
- Ring of smooth muscle
- Innervation:
- Parasympathetic ⇒ pelvic splanchnic nerves
- Sympathetic ⇒ hypogastric nerves
- Sympathetic control most significant
External Urethral Sphincter
(EUS)
- Composed of striated muscle
- Innervated by the somatic nervous system
- Motor neuron’s originate in Onuf’s nucleus
- Traverse the pudenal nerve
- Allows voluntary excretion of urine
Bladder Body
Innervation
Parasympathetic Innervation
Stimulates detrusor contraction.
- Body mostly innervated by M2 and M3 receptors
- M2 receptors more abundant
- M3 receptors most significant
-
M3 mediates the majority of bladder contraction
- functions via IP3-gated Ca2+ channels
- release of intracellular Ca2+ results in activation of MLC kinase ⇒ contraction
Sympathetic Innervation
Inhibits bladder contraction.
- Body also has 𝛽2 and 𝛽3 adrenergic receptors
- Binding of NE results in detrusor reflaxation
- Via stimulation of adenylyl clycase ⇒ cAMP
- Facilitates urine storage
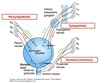
Bladder Neck
Innervation
Richly innervated by sympathetic nervous system.
Thoracolumbar spine ⇒ hypogastric nerves.
- Abundant 𝛼1 receptors
- Binding of NE stimulates contraction of bladder neck
- Via IP3/DAG pathway
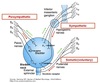
Urethral Innervation
Internal Urethral Sphincter
Sympathetic innvervation
Hypogastric nerve ⇒ NE ⇒ 𝛼1 receptors ⇒ IP3 ⇒ Ca2+ ⇒ contraction
External Urethral Sphincter
Somatic Innervation
Pudenal nerve ⇒ Ach at NMJ ⇒ nicotinic receptors ⇒ Ca2+ ⇒ contraction

Bladder
Efferent Innervation
Summary

Bladder
Afferent Innervation
LUT afferent axons found in the:
Pelvic splanchnic nerve
Hypogastric nerve
Pudenal nerve
Pelvic splanchnic nerves most important.
Relays information about bladder filling and noxious stimuli.
LUT Coordination
Proper functioning of LUT relies on reciprocal relationship between bladder and urethral activity.
-
Storage
-
bladder body contractility low
- SNS relaxation
-
outlet resistance high
- SNS contraction of bladder neck and urethra
- somatic contraction of EUS
- little change in intravesical pressure between 100 and 400 mls
-
bladder body contractility low
-
Micturition
-
bladder body contractility high
- PNS ⇒ muscarinic receptors ⇒ relaxation
-
outlet resistance low
- inhibition of somatic and sympathetic systems
- PNS ⇒ NO ⇒ relaxation
- Relies on spino-bulbo-spinal reflexes from rostral pons
- Voluntary control via supra-pontine regions of brain to micturition control center
-
bladder body contractility high

Dyssynergia
Lack of coordination of bladder and urethral sphincter activity.
- Bladder contraction against a closed sphincter
- Interferes with bladder emptying
- Often seen with spinal cord injury above S4
- descending inhibition of bladder-to-EUS spinal storage reflex interrupted
- Bladder may develop hyperreflexia
- frequent, high pressure contractions
Urine Storage
Spinal Reflex
Primarily involves spinal reflexes.
Pontine Storage Center can modulate them.
-
Afferent Pathway
- Low level of afferents via pelvic splanchic nerve
- In response to minimal bladder stretch
- Compliance & passive properties of bladder smooth muscle
-
Efferent Pathways
- EUS contraction via pudendal nerve
- IUR contraction via SNS
- Inhibition of detrusor muscle contraction via SNS
- PNS to detrusor inactive
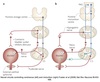
Guarding Reflex
Increased external urethral sphincter activity in response to an increase in intravesical pressure.
Micturition
Spinobulbospinal Reflex
When micturition threshold reached, LUT switches from storage phase to voiding phase.
Voiding can be consciously controlled via cortical input to micturition center.
Fluid flow through urethra reflexively causes detrusor contraction and EUS relaxation.
-
Afferent pathways:
- High levels of vesical afferent activity via pelvic splanchnic nerve
- Due to higher levels of bladder stretch
-
Efferent pathways:
- Inhibition of EUS
- Inhibition of SNS ⇒ bladder and urethra
- Activation of PNS ⇒ bladder and urethra
Results in relaxation of urethral sphincters & contraction of bladder.
Bladder pressure increases leading to urine flow.
Modulation by cerebral cortex permits voluntary voiding.
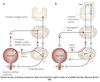
Effects of Intravesical Pressures
-
At 100-150 ml:
- Activation of stretch receptors in bladder wall
- Impulses sent to sacral spinal cord
- Spinobulbospinal reflex stimulated
- First sensation of bladder filling
-
At 150-300 ml:
- Bladder contracts and IUS relaxes through end of reflex arc
-
Cortex senses urge to void & proper location
- Can allow voluntary EUS relaxation and excretion
-
At 400 ml:
- Intravesical pressure increases sharply
- Partially due to reflex contractions of detrusor
-
At 700 ml:
- Pain develops
- Loss of sphincter control



