Paper1-SC14/SC15/SC16 Flashcards
What is the equation for ammonium sulfate?

Explain the apparatus for a hydrogen pxygen fuel cell.

What factors are taken into account when choosing a reaction pathway?
Availability and cost of raw materials, the rate of reaction and equilibrium position and atom economy and yield and usefulness of by-products.
What is the other equation for concentration in mol dm?

What is the equation for atom economy?

How is the energy transferred in chemical cells?
Mainly electricity but a bit of heating.
What are titrations used to find?
Exact amount of acid that neutralises a specific volume of alkali. We use indicators that are not universal so the change is not graduate and it is like big step.
What is ammonium nitrate?
Nitrogen rich fertiliser(nitrogenous fertiliser).
Explain the apparatus for a chemical cell?

Why do batteries go flat?
When one of the reactants is used up the reaction stops and voltage is no longer produced.
What is the equation for the amount of gas(mol)?
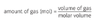
What type of process is the laboratory preparation of ammonium sulfate?
Batch process, whereas the industrial preparation is continuous.
Where is a solution with an accurate concentration made?
In a volumetric flask which are calibrated to measure one volume of solution.
What is the equation for concentration in mol dm?

What happens in a dynamic equilibrium?
Forwards and backwards reaction happen at same rate and the concentrations of all reacting substances do not change.
What is the equation for concentration in mold dm?

How can you convert cm to dm?
Divide by 1000.
What components are used in chemical cells?
Two different metals each dipped into a solution of one of their salts. A salt bridge to allow dissolved ions to pass from one solution to another.
What does avogrado’s law state?
If the temperature and pressure are the same. equal volumes of different gases contain an equal number of molecules.
What is the equation for percentage yield?

What are the strengths and weaknesses of fuel cells?
They do not produce carbon dioxide, however most hydrogen is manufactured by the reaction of steam with coal or natural gas which releases carbon dioxide as a by-product. Very hard tp stpre hydrogen and can be dangerous if released(simple and light so hard to store).
What is atom economy?
Method of showing how efficient a particular reaction makes use of the atoms in the reactants.
What is another equation for the concentration in g dm?

What is the mole ratio?
Ratio in moles of the substance in a balanced equation.







