Mod VI: Congenital Heart Disease Flashcards
Congenital Heart Disease
Congenital Heart Diseases are present in about what percentage of newborn infants?
1%
Congenital Heart Disease
What are the causes of Congenital Heart Disease?
Idiopathic
Genetic
Environmental
(rubella 1st trimester, lithium, FAS)
Congenital Heart Disease
What are risk factors for Congenital Heart Disease?
Parent with CHD
Prematurity
Multiple gestations
Noncardiac congenital anomalies (Down’s syndrome)
Congenital Heart Disease
Signs & Symptoms of Congenital Heart Disease in infants are:
Tachypnea
Failure to gain weight
Tachycardia (>200)
Heart murmur
Congestive heart failure
Hypoxemia
Cyanosis
Congenital Heart Disease
Signs & Symptoms of Congenital Heart Disease in children are:
Dyspnea
Failure to grow
Decreased exercise tolerance
Heart murmur
Congestive Heart Failure
Cyanosis
Clubbing of digits
Squatting (To increase SVR)
HTN
Chest pain
Congenital Heart Disease
T/F: Most Congenital Heart Diseases are diagnosed prior to birth
True
Congenital Heart Disease - Diagnosis
T/F: Congenital Heart Disease is apparent during first week of life in 50% of afflicted neonates and before 5yrs in all remaining
True
Congenital Heart Disease - Diagnosis
What’s the initial diagnostic test recommended for CHD?
US Echocardiography
Congenital Heart Disease - Diagnosis
Test that demonstrates valvular dysfunction and septal defects
Doppler US
Congenital Heart Disease - Diagnosis
Tests that demonstrate anomalies involving great vessels
CT scan - MRI
Congenital Heart Disease - Diagnosis
What’s the most definitive diagnostic technique for CHD?
Cardiac catherization
Congenital Heart Disease
Problems afflicting patients with Congenital Heart Disease include:
Pulmonary vascular disease & associated PHTN
Congestive heart failure
Infective endocarditis (VSD/PDA)
Requires prophylaxis antibiotics
Hypertension (Coarctation)
Polycythemia (HCT > 65%)
Physiologic response to chronic hypoxemia - Increases risk for thromboembolism
Coagulation defects
Deficiency in VT K clotting factors - Defective PLT aggregation
Brain abscess development
Problems Afflicting Patient with Congenital Heart Disease
Congenital Heart Disease a/w Infective endocarditis (VSD/PDA) Requires Prophylaxis with which drugs?
Antibiotics
Problems Afflicting Patient with Congenital Heart Disease
Polycythemia (HCT > 65%) a/w Congenital Heart Disease is a physiologic response to:
Chronic hypoxemia
Problems Afflicting Patient with Congenital Heart Disease
Polycythemia (HCT > 65%) a/w Congenital Heart Disease increase risk for:
Thromboembolism
Problems Afflicting Patient with Congenital Heart Disease
Coagulation defects a/w Congenital Heart Disease are a consequence of:
Deficiency in Vit K clotting factors
Defective PLT aggregation
Pathophysiology of Congenital Heart Disease
T/F: Management of anesthesia for patients with CHD requires a thorough knowledge of the pathophysiology of each cardiac defect
True
However, this is confusing due to complexity of lesions
Utilization of a structured approach that emphasizes ratio of pulmonary blood flow & systemic blood flow based on resistance in these vascular beds is helpful
Pathophysiology of Congenital Heart Disease
Important pathophysiologic questions w/ CHD include:
Is there on obstruction?
Is there a shunt?
Pathophysiology of Congenital Heart Disease
What are the effects of R side obstruction?
Blood unable to go from RV to lungs
↓ pulmonary blood flow => hypoxemia/cyanosis
Blood does not get oxygenated
Pathophysiology of Congenital Heart Disease
What are effects of L side obstruction?
Blood unable to flow from LV to systemic circulation
Tissues organs do not get perfused
↓ systemic blood flow => hypoperfusion/acidosis/shock
Pathophysiology of Congenital Heart Disease
How can shunt be defined?
Mixing of pulmonary/systemic circulations
(or mixing of oxygenated and de-0xygenated blood)
Pathophysiology of Congenital Heart Disease
What determines the direction of of shunt?
Ratio of pulmonary blood flow (Qp) / systemic blood flow (Qs)
Qp:Qs
Pathophysiology of Congenital Heart Disease
Qp:Qs < 1 means:
Pulmonary blood flow < Systemic blood
Instead of flowing to the lungs, blood is flowing to the left side
[R to L shunt]
Blood flowing directly to the left fails to be oxygenated
This leads to hypoxemia and cyanosis
Ineffective pulmonary blood & mixing systemic/pulmonary circulations => hypoxemia/cyanosis
Pathophysiology of Congenital Heart Disease
Qp:Qs > 1 means:
Pulmonary blood flow > Systemic blood
[L to R shunt]
Volume/pressure overload of R ventricle => CHF
Pulmonary overcirculation => Pulmonary HTN/ ↑ PVR
Pathophysiology of Congenital Heart Disease
Qp:Qs = 1 means
No shunt
Balanced flow
Bi-directional shunt of equal magnitude
Pathophysiology of Congenital Heart Disease
Shunt flow dependent on balance between PVR & SVR. ↑ PVR relative to SVR would lead to what shunt direction?
R to L shunt
Pathophysiology of Congenital Heart Disease
Shunt flow dependent on balance between PVR & SVR. ↑ SVR relative to PVR would lead to a shunt in which direction?
L to R shunt
Pathophysiology of Congenital Heart Disease
Factors that would increase PVR, cause a R-to-L shunt, and affect Qp:Qs Ratio include:
Hypoxia
Hypercapnia
Acidosis
High PIP
PEEP
Hypothermia
Polycythemia
Decreased LV output
Pathophysiology of Congenital Heart Disease
Factors that would decrease PVR, cause a L-to-R shunt, and affect Qp:Qs Ratio include:
High FiO2
Hypocapnia
Alkalosis
Improved LV output
Anemia
Classification of Congenital Heart Defects
Lesions causing left-to-right shunting (volume overload of the left ventricle or left atrium resulting in increased pulmonary blood flow) include:
Atrial Septal Defect
Ventricular Septal Defect
Patent ductus arteriosus
Atrioventricular Septal Defect
(Common complete atrioventricular canal)
Classification of Congenital Heart Defects
Lesions causing outflow obstruction (resulting in pressure overload on the left ventricle, and increased myocardial work) inlude:
Aortic Stenosis
Coarctation of the aorta*
(Narrowing of the aorta distal to the aortic valve)

Classification of Congenital Heart Defects
Lesions causing right-to-left shunting (cyanosis resulting from obstruction/decreased pulmonary blood flow) include:
Tetralogy of Fallot
Tricuspid atresia
Pulmonary atresia
Classification of Congenital Heart Defects
Lesions causing right-to-left shunting (cyanosis due to mixing of the pulmonary and systemic circulations/increase pulmonary blood flow) include:
Hypoplastic left heart syndrome
Truncus arteriosus
Classification of Congenital Heart Defects
Lesions causing separation of the pulmonary & systemic circulations
Transposition of the great vessels
Classification of Congenital Heart Defects
Acyanotic (L→R) shunt lesions include:
Ventricular Septal Defect
Atrial Septal Defect
Patent Ductus Arteriosus
Atrioventricular Septal Defects
Common Complete Atrioventricular Canal
Aortic Stenosis
Coarctation of the Aorta
Classification of Congenital Heart Defects
Cyanotic (R→L) shunt lesions include:
Tetralogy of Fallot
Transposition of the Great Arteries
Hypoplastic Left Heart Syndrome (HLHS)
Tricuspid valve abnormalities (Ebstein’s anomaly)
Truncus arteriosus
Total anomalous pulmonary venous connection
Acyanotic or Predominantly Left-to-Right Lesions
Pathophysiologic changes that accompany Left-to-Right shunt lesions include:
Decreased systemic blood flow
Low Cardiac output - Hypotension
Increased pulmonary blood flow
Pulm HTN - RVH

Acyanotic or Predominantly Left-to-Right Lesions
Hemodynamic goals for Left-to-Right shunt lesions include:
Avoid increased SVR
—
Avoid decreased PVR
How? => Decrease FiO2 - Hypoventilation
(High FiO2 and Hypocapnia/hyperventiation will decrease PVR)
Any of the above will worsen the shunt!!!

Acyanotic or Predominantly Left-to-Right Lesions
Pathophysiology of Left-to-Right Lesions:
Simple shunts with isolated abnormal communications between the L & R side of the heart
Pressure L side heart > Pressure R side heart => L-to-right shunt
Blood flow to R side heart & lungs increases
Shunt flow depends on balance between PVR & SVR
PVR < SVR => L-to-R shunting

Acyanotic or Predominantly Left-to-Right Lesions
Clinical manifestations:
Pulmonary vascular congestion
Decreased lung compliance
Increased work of breathing
Chronic increases PBF
Irreversible ↑ PVR
RVH → Cor Pulmonale

Acyanotic or Predominantly Left-to-Right Lesions
What’s the most common acyanotic or predominantly Left-to-Right Lesion?
Ventricular Septal Defect (VSD)
Most common (25%)
Mostly “isolated” VSD = VSD w/o any other type of anomalies
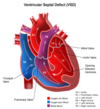
Acyanotic (L→R) - Ventricular Septal Defect (VSD)
Clinical symptoms related to size of shunt - what are Clinical symptoms of Small shunt?
No clinical symptoms

Acyanotic (L→R) - Ventricular Septal Defect (VSD)
Clinical symptoms related to size of shunt - what are Clinical symptoms of Large shunt?
Growth failure
CHF
Recurrent pulmonary infections
Eisenmenger’s syndrome (untreated)
Acyanotic (L→R) - Ventricular Septal Defect (VSD)
Condition that arises when untreated Ventricular Septal Defect (VSD) leads to Pulmonary vascular disease => ↑ PVR, and Shunt reverses and flows R-to-L (cyanosis)
Eisenmenger’s syndrome
Deadly during delivery or shortly after birth d/t severe cyanosis, b/c the body is unable to compensate for the acute change from L to R to R to L shunt flow
Acyanotic (L→R) - Ventricular Septal Defect (VSD)
Shunt flow is determine by PVR & SVR. How does ↓ PVR & ↑ SVR affect an existing L-to-R shunt?
↑ L-to-R shunting
(Makes it worse)

Acyanotic (L→R) - Ventricular Septal Defect (VSD)
Shunt flow is determine by PVR & SVR - How does ↑ PVR & ↓ SVR affect an existing L-to-R shunt?
↓ L-to-R shunting

Acyanotic (L→R) - Ventricular Septal Defect (VSD)
T/F: Most small Acyanotic (L→R) defects close w/o intervention
True
(40% by 3yrs and 75% by 10yrs)

Acyanotic (L→R) - Ventricular Septal Defect (VSD)
How are Large Acyanotic (L→R) defects treated?
Require surgical closure before ↑ PVR irreversible

Acyanotic (L→R) - Ventricular Septal Defect (VSD)
Anesthetic considerations similar to which other congenital heart defect?
ASD
Acyanotic (L→R) - Ventricular Septal Defect (VSD)
Blood flow in VSD
See picture

Acyanotic (L→R) - Atrial Septal Defect (ASD)
Prevalence of ASD
Accounts for 7.5% of CHD

Acyanotic (L→R) - Atrial Septal Defect (ASD)
T/F: Most small Atrial Septal Defect (ASD) are asymptomatic, with spontaneous closure occurring in the 1st yr. of life
True

Acyanotic (L→R) - Atrial Septal Defect (ASD)
Pathophysiology of large ASD
Acyanotic L to R shunt (pulmonary overflow)

Acyanotic (L→R) - Atrial Septal Defect (ASD)
Symptoms of Atrial Septal Defect (ASD)
DOE - SVT - CHF
Pulmonary HTN
Recurrent pulmonary infections

Acyanotic (L→R) - Atrial Septal Defect (ASD)
Treatment of large Atrial Septal Defect (ASD)
Requires surgical repair or
Placement of a device via catheterization

Acyanotic (L→R) - Atrial Septal Defect (ASD)
Blood flow in Atrial Septal Defect (ASD)
See picture

Acyanotic (L→R) - Atrial Septal Defect (ASD)
Anesthetic considerations for ASD and VSD will focus on avoiding which changes to which two hemodynamic variables?
Avoid ↑ SVR
(worsen L-R shunting)
Avoid ↓ PVR
(by avoiding high FiO2, and avoiding low ETCO2)
Acyanotic (L→R) - Atrial Septal Defect (ASD)
T/F: Anesthetic considerations for ASD and VSD include Strict avoidance of air emboli
True
Acyanotic (L→R) - Atrial Septal Defect (ASD)
How does the L-to-R shunting affect the rate of inhalation induction? why?
More rapid with inhalation induction
D/t _rapid decrease in the arterial to venous difference of agen_t
This is all theoretical - No real differences seen clinically
Acyanotic (L→R) - Atrial Septal Defect (ASD)
How does the L-to-R shunting affect the rate of IV induction? why?
Slower rate of IV inductions
D/t Diluted arterial blood concentration
This is all theoretical - No real differences seen clinically
Acyanotic (L→R)
Failure of ductus arteriosus to close after birth (review fetal circulation and transition at birth!) results in a condition called:
Patent Ductus Arteriosus (PDA)

Acyanotic (L→R) - Patent Ductus Arteriosus (PDA)
What’s the prevalence of PDA? What age group is most affected?
PDA accounts for 7.5% CHD
Most common in the premature infants

Acyanotic (L→R) - Patent Ductus Arteriosus (PDA)
What are the causes of Patent Ductus Arteriosus (PDA)?
Hypoxemia
Hypercarbia/acidosis
Persistent pulmonary HTN in the newborn

Acyanotic (L→R) - Patent Ductus Arteriosus (PDA)
Which hemodynamic variables determine shunt flow in Patent Ductus Arteriosus (PDA)?
SVR & PVR
PDA is Nonrestrictive when SVR > PVR
Which results in L-to-R shunt
(blood flow from aorta back into pulmonary artery

Acyanotic (L→R) - Patent Ductus Arteriosus (PDA)
Blood flow in PDA
See image

Acyanotic (L→R) - Patent Ductus Arteriosus (PDA)
Clinical presentation of PDA:
Pulmonary congestion
CHF
(widened pulse pressure, continuous systolic/diastolic murmur)

Acyanotic (L→R) - Patent Ductus Arteriosus (PDA)
How long after birth should PDA be normally closed?
2-3 days after birth

Acyanotic (L→R) - Patent Ductus Arteriosus (PDA)
Medical Treatment of Patent Ductus Arteriosus (PDA)
Indomethacin (↓ PGE1 levels)
Remember that it is the reduction in prostaglandins production after removal of the placenta that results in the closure of the ductus arteriosus
Indomethacin reduces levels of prostaglandins (PGE1)
Acyanotic (L→R) - Patent Ductus Arteriosus (PDA)
When is Surgical ligation (NICU/Cath lab) of Patent Ductus Arteriosus (PDA) indicated?
Decreased renal or platelet function
(contraindication to indomethacin use)
Indomethacin unsuccessful
Decreased systemic oxygenation due to shunting
Acyanotic (L→R) - Patent Ductus Arteriosus (PDA)
What surgical approch is used for ligation (NICU/Cath lab) of Patent Ductus Arteriosus (PDA)?
Left thoracotomy approach
Video-assisted ligation (minimally traumatizing)
Acyanotic (L→R) - Patent Ductus Arteriosus (PDA)
What’s a contraindication to indomethacin use?
Decreased renal or platelet function
Acyanotic (L→R) - Patent Ductus Arteriosus (PDA)
Anesthetic considerations for PDA are similar to Anesthetic considerations for which other Acyanotic (L→R) lesions?
ASD/VSD
Avoid increasing SVR => this will worsen the shunt
Avoid decreasing PVR => by avoiding high FiO2 and hyperventilation
Acyanotic (L→R)
Which anatomical defects are present in Atrioventricular Septal Defects?
ASD
VSD
Single atrioventricular valve
Lack of separation of the mitral and tricuspid valves

Acyanotic (L→R) - Atrioventricular Septal Defects
Atrioventricular Septal Defects are common in children with which genetic condition?
Trisomy 21
(Down syndrome)

Acyanotic (L→R) - Atrioventricular Septal Defects
What are the characteristics of Shunt flow w/ AVSD in the initial neonatal period? why is that and what does that results in?
Bidirectional
D/t ↑ PVR
Results in mild hypoxemia

Acyanotic (L→R) - Atrioventricular Septal Defects
How is Shunt flow in AVSD with ↓ PVR
Predominantly L to R

Acyanotic (L→R) - Atrioventricular Septal Defects
What are the Symptoms of AVSD?
CHF
Tachypnea/dyspnea
Poor feeding
Pulmonary HTN
(with <u>pulmonary vascular disease</u> developing overtime)

Acyanotic (L→R) - Atrioventricular Septal Defects
What’s the Treatment for AVSD?
Surgical repair required within the 1st year of life

Obstructing Lesions
What’s the prevalence of Congenital Aortic Stenosis?
Accounts for 5% of all CHD

Obstructing Lesions - Congenital Aortic Stenosis
Anatomic and physiologic changes a/w congenital Aortic Stenosis include:
Unicuspid or bicuspid stenotic valve
↑ LVEDP & ↑ LAP => Pulmonary edema
L to R shunt at atrial level
Concentric LVH with_↑ Myocardial O2 requirements_

Obstructing Lesions - Congenital Aortic Stenosis
T/F: Symptoms of Congenital Aortic Stenosis are related to severity of stenosis and ventricular function
True

Obstructing Lesions - Congenital Aortic Stenosis
Systemic blood flow in neonate with critical Congenital Aortic stenosis is dependent on:
Maintaining a Patent Ductus Arteriosus
Ductal-dependent systemic blood flow (R-L shunting)
Closure of PDA after birth => cardiogenic shock

Obstructing Lesions - Congenital Aortic Stenosis
What’s the treatment for critical Congenital Aortic stenosis in Neonate?
Prostaglandin E1 to maintain open ductus arteriosus until definitive surgery can be performed

Obstructing Lesions - Congenital Aortic Stenosis
Congenital Aortic Stenosis is most commonly diagnosed in older children. What s/s is it a/w?
Angina pectoris w/o CAD (LVH)
CHF
Syncope

Obstructing Lesions - Congenital Aortic Stenosis
In Critical AS, the aortic valve opens, but cannot supply enough blood to the body. Some part of the blood supply to the body must be supplied by
A Patent Ductus Arteriosus

Obstructing Lesions - Congenital Aortic Stenosis
Surgical intervention for Congenital Aortic Stenosis include
Valvuloplasty/Valvotomy
Valve replacement
Obstructing Lesions - Congenital Aortic Stenosis
Why do Surgical intervention for Congenital Aortic Stenosis include require prophylactic antibiotics?
These pts are predisposed to infective endocarditis
Obstructing Lesions - Congenital Aortic Stenosis
Anesthetic management of peds with Congenital Aortic Stenosis is the same as for the adult patient with aortic stenosis. What does it entail?
Avoid sudden↓ SVR
Maintain NSR
Avoid bradycardia (↓ C.O.)
Avoid tachycardia (impairs ventricular filling)
Optimize volume to maintain venous return & LV filling pressures
Predisposed to infective endocarditis => prophylactic antibiotics
Avoid hyperoxygenation
↑PBF thus reducing systematic blood flow
Accept SaO2 > 75%
Reduce FiO2 SaO2 > 85%
Maintain Controlled hypoventilation (↑ PVR)
If there is excessive PBF (SaO2 > 85%) & reduced SBF (↓ MAP)
Obstructing Lesions - Congenital Aortic Stenosis
In the Anesthetic management of peds with Congenital Aortic Stenosis, why must hyperoxygenation be avoided?
Decreases PVR => ↑ pulm blood flow (PBF)
=> reduced systematic blood flow
Accept SaO2 > 75%
Reduce FiO2 SaO2 > 85%
Obstructing Lesions - Congenital Aortic Stenosis
In the Anesthetic management of peds with Congenital Aortic Stenosis, which intervention is appropriate if there is excessive PBF (SaO2 > 85%) & reduced SBF (↓ MAP)?
Controlled hypoventilation (to ↑ PVR)


