Lecture Twenty Two - Crystal field theory II Flashcards
(5 cards)
How does a coordination compound change shape depending on the ligands it is bonded to?
Where two ligands are chemically different from the other four, distortions from octahedral can arise.
E.g. [CoCl2(NH3)4]+.
Orbital energies will change correspondingly.
If axial ligands are further removed, we get the orbital splitting for a square planar complex.
In square planar complexes, the dz^2 orbital has a much lower energy.
This is because electrons no longer repel one another and therfore

Explain Hunds rule in relation to coordination complexes.
Splitting of energy levels influences number of unpaired electrons.
Hund’s rule - electrons prefer to go into different orbitals of same energy - pairing of electrons is energetically unfavourable.
When lower levels are half filled, the next electron can go into:
- A half filled orbital and pair up (overcomes repulsive energy).
- An emptie higher energy orbital (overcomes splitting energy difference).
Explain magentism in transition metals.
So how can we determine the number of d electrons and whether of not the metal complex is high spin or low spin?
Firstly, we can count the number of unpaired electrons if we know the metal ion, the oxidation state and the geometry.
Paramagnetism and diamagnetism:
It is possible to experimentally determine the number of unpaired electrons, n, by measuring the effective magnetic moment, μeff for the complex.
To do this, we place the compound in a magnetic field; diamagnetic (paired electrons) are repelled by the magnetic field,
paramagnetic (unpaired electrons) are attracted to the field.
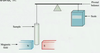
This is done using a Gouy Balance.
Gouy Balence:
The sample is placed in a glass tube and weighed with and without the magnetic field.
Paramagnetic compounds are attracted in to the field, the extent of attraction depends on the number of unpaired electrons, n.
The more unpaired electrons, the higher the attraction.
Diamagnetic = Weight increases.
Paramagnetic = Wight decreases.
Explain the effective magnetic moment.
From the attraction causing a change in weight of the sample, we calculate the magnetic susceptibility, χ which is used to calculate the effective magnetic moment, μeff using;
μeff = 2.828 (χM’ x T)^1/2
where:
μeff = effective magnetic moment, Bohr magneton, B.M.
χM’ = molar magnetic susceptibility (corrected). T = in Kelvin, ie. K.
Note: Units are non-SI for magnetochemistry.
What is the spin only formula?
Paramagnetism arises from the negatively charged electrons spinning & circulating around the nuclei. This generates a magnetic field.
Two effects: electron spin & orbital angular momentum.
For first row transition metal ions the dominant contribution is the electron spin.
⇒ spin-only magnetic moment, μSO = √n(n+2) (where n = numbers of unpaired electrons).
E.g.
High spin octahedral. Cr2+, (d4 ion).
μSO =√n(n+2)=√4x6=√24=4.90B.M.
cf. measured value μeff ~ 4.7-4.8 B.M.


