Lecture Four - Reaction mechanisms (substitution, REDOX) and aromaticity Flashcards
(14 cards)
What do primary, secondary and tertiary carbocations look like?

How is an alcohol converted to an alkyl halide?
This involved substitution of the halogen for -OH. The most common reagents for this process are the halogen acids, HX and thionyl chloride, SOCl2.
Tertiary alcohols react very rapidly with HCl, HBr and HI.
Low molecular weight primary and secondary alcohols are unreactive under these conditions.

Are alkyl halides suseptible to nucleophiles or electrophiles?
Alkyl halides are suseptible to nucleophiles.

What are some interconversions between functional groups?

What is an oxidation reaction?
Occurs formally with the loss of electrons from an atom or molecule.
Loss of electrons.
Loss of hydrogen.
Gain of oxygen.
In organic chemistry, this statement can be generalised as the addition of oxygen to (typically) a molecule.

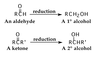
How does the oxidation of alcohols work?

What is a reduction reaction
Occurs formally with the gain of electrons from an atom or molecule.
Gain of electrons.
Gain of hydrogen.
Loss of oxygen.
In organic chemistry, we can generalise this statement as the addition of two hydrogen atoms to (typically) an unsaturated hydrocarbon, aldehyde, ketone or carboxylic acid derivitive.
Activated metals are used to catalyse reductions of carbonyl groups. E.g. Pt at 25 degrees C and 2 atm. E.g. Ni and 2H2.
An aldehyde can be reduced to a primary alcohol, and a ketone to a secondary alcohol.
What is the thermochemical evidence for the non-triene-like nature of benzene?
Evidence suggests that benzene should not be described as a triene with localised single and double C-C bonds.
This evidence consisted of the fact that less energy than though was required to break the bonds between 3H2 atoms when added to benzene.

Does benzene react like an alkene?
NO.
What are the different ways in which benzene can be drawn?

Describe the delocalised bonding model of benzene.
Hybridization of atomic orbitals and the resonance theory provided the first adequate description of benzenes structure and reactivity.
The carbon skeleton is a regular hexagon, with all C-C-C and H-C-C bond angles at 120 degrees (VB model).
Each carbon is sp2 hybridised with one p orbital containing one electron.
Overlap of the six parallel 2p orbitals formes a ‘donut-shaped’ continuous pi cloud.

What is aromaticity? What is the Huckel rule?
A special characteristic associated with the planar ring systems with a delocalised p-system.
Allows rectivity and structure of benzene and related structures to be understood.
Huckel rule:
A compound with a planar ring system and a delocalised pi-system containing (4n+2) pi-electrons, when n is an integer.
Includes neutral molecules, anions and cations is aromatic.

What is Phenol (hydroxybenzene)?
Phenols are compounds in which an OH group is attached directly to an aromatic ring.
Phenol is a colourless crystalline solid. Soluble in water and a wide range of organis solvents.
The OH group in phenol pushes electron density onto the ortho- and para- positions.
The OH group is strongly activating and ortho- and para- directing.
What is aniline (aminobenzene)?
Aromatic primary amine behaves as a very weak base.
Aniline is a colourless, oily liquid. Slightly soluble in water.
Weak base resonance ‘spreads’ the electron density.
The NH2 group is highly activating and ortho- and para - directing.
Electrophilic substitution with halogens occurs readily and multi-substituted products are obtained.


