Lecture Three - Reaction mechanisms and addition reactions Flashcards
(15 cards)
What is reaction mechanism?
Reaction mechanism describes the formation and breakage of bonds.
How do the curley arrows work for the formation and breakage of bonds?
A+ is an electrophile and B- is a nucleophile.
A single sided head of an arrow represent the movement of one electron.
A two sided (normal looking) arrow represents the movement of two electrons.

What are the five rules for the making and breaking of bonds?
Electrons go from an electron rich atom to an electron poor atom.
1) Have the same number of valance electrons after the making or breaking of bonds, as there were to begin with.
2) Obey the rules of covalent bonding.
3) Differ only in distribution of valance electrons.
4) Have the same number of paired and unpaired electrons.
5) Have a charge balence follwing the redistribution.
Define nucleophiles and electrophiles.
Nucleophile: Are electron rich species that donate an electron pair to an electrophile. They can be neutral or negitively charged. Generally acids. Usually have lone pairs.
Electrophile: Are electron poor specie that accept an electron pair from a nucleophile. They can be nutral or positively charged. Generally bases.
What is an addition reaction?
One where an atom or group of atoms is added (formally or informally) to a molecule without the loss of any other atom or group of atoms.
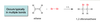
What does regioselective mean?
One direction fo bond-forming or bond breaking occurs in preference to all other directions.
Additions are regioselective.
What substances can under go addition reactions?
Additions are carried out with pure reagents or in a polar solvent such as acetic acid.
What is Markovnikov’s rule?
In additions of HX to alkenes, H adds to the carbon with the greater number of hydrogens.
Write out the two step mechanism of HCl + 2-Butene.

Draw an energy diagram for the two step addition of HCl to 2-butene.
The deeper the well, the more stable something is.
Tertiary carbocatios are far more stable than secondary and primary.

Define a carbocation.
A species in which a carbon atom has only six electrons in it valance shell and bears positive charge.
Carbocations are:
1) Classified as 1 degree, 2 degrees or 3 degrees depending on the number of carbons bonded to the carbon bearing the positive charge.
2) Lewis acids - that is, they are electron-pair accepters.

What are the carbocation stability trends?
A tertiary (3 degrees) carbocation is more stable than a secondary carbocation, adn requires a lower activation energy for its formation.
A secondary (2 degrees) carbocation is, in turn, more stable than a primary carbocation, and requires a lower activation energy for its formation.
What are stereoselective reactions?
Those in which one stereoisomer is formed or destroyed in preference to all other that might be formed or destroyed.
E.g. When cyclohexan is added to Br2, the Bromine atoms always bond on opposite sides of the cyclohexane, as they are quite large atoms, and can’t be bonded any closer.

Explain hydration.
The addition of water is called hydration.
Acid-catalyzed hydration of an alkene is regioselective - hydrogen adds preferentially to the less substituted carbon of the double bond.

What are some characteristic addition reactions?



