Lecture notes/slides Flashcards
(127 cards)
What is neuroscience?
Neuroscience is the study of all aspects of nervous system function from molecular to cellular to systems to cognitive (behavioral). The goal is to integrate across all of these levels.
Neurons (basic)
Neurons function to receive, integrate and transmit information. There are ~ a hundred billion neurons in the human brain. Neurons receive an average of ~ 5,000 synaptic contacts.
Glia (basic)
The term glia means glue. 2. There are three types of glia: a. Oligodendrocytes and Schwann cells wrap around the axon to provide insulation in the form of myelin. Oligodendrocytes are found in the central nervous system (CNS) and Schwann cells are found in the peripheral nervous system (PNS). b. Astrocytes provide supporting function for neurons. Astrocyte processes wrap neuronal synapses. They regulate neuronal excitability by buffering extracellular potassium (K+) ions and taking up glutamate released by neurons via glutamate transporters. Astrocytes may also provide metabolic support for neurons via endfeet that wrap the cerebrovasculature.  c. Microglia are CNS resident immune cells (phagocytes) that become activated during infection and clean up cellular debris produced by damage. 3. There are ~ (approximately) ten times more glia than neurons.
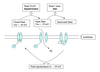
Basic structure of a neuron
- Soma
2. Nucleus - Dendrites
- Axon hillock
- Axon
- Myelin (formed by oligodendrocytes [CNS] or Schwann cells [PNS])
- Node of Ranvier
- Axon Collateral
- Presynaptic terminal (contains synaptic vesicles)
- Synaptic Vesicles (contain molecules of neurotransmitter)
- Synaptic cleft
- Postsynaptic density (site of receptors)

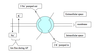
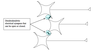
Functional zones of a neuron (schematic)

Functional zones of a neuron (chart)

What is the average resting membrane potential?
-65mV
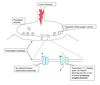
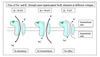
Image of hyperpolarization and depolarization
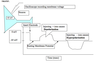
Hyperpolarization vs depolarization
As used in neuroscience Depolarization means - the membrane voltage becomes more positive.
As used in neuroscience Hyperpolarization means - the membrane voltage becomes more negative.
1. Positive ion influx results in a depolarization.
2. Positive ion efflux results in a hyperpolarization.
- Negative ion influx results in a hyperpolarization.
- Negative ion efflux results in a depolarization.
What is the neuronal membrane?
The neuronal membrane is a lipid bilayer composed of phospholipids (text figure 3.3).
- The polar phosphate heads are hydrophilic.
- The nonpolar lipid tails are hydrophobic.
- Charged ions are hydrated (surrounded by water molecules). Therefore they are attracted to hydrophilic regions and repelled by hydrophobic regions. Thus ions cannot pass through the neuronal membrane and this is what maintains the separation of charges that is essential to the RMP.
What is an ion channel?
An ion channel is a protein with a pore through which ions can flow.
2. The pore has open and closed states, with the default state (no stimulation) being closed.
3. Different types of ion channels are permeable to different ions (i.e., some are permeable to sodium, some to potassium, some to chloride, some to calcium and some to multiple ions). Thus, there are sodium channels, potassium channels, chloride channels etc.
What are the main classe of ion channels?
- Ligand-gated (a.k.a. neurotransmitter-gated) ion channels (a.k.a. neurotransmitter receptors).
a. The binding of the ligand (the neurotransmitter) to the receptor causes the ion channel pore to open.
b. Each type of receptor specifically binds only one type of neurotransmitter. Note for future reference: There are also chemical receptors that are not ion channels. - Voltage-gated ion channels.
a. These channels are opened and closed by changes in the voltage across the membrane. The opening and closing of these channels is dependent upon the amplitude and direction of the voltage change. - Another type of ion channels are the leak channels.
a. These channels are not gated by either voltage changes or neurotransmitters.
b. An example of a leak channel is the K+ leak channel.
c. The default state of a leak channel is open! (opposite to the other types of
channels) .
K+ leak channel
A. The K+ leak channel is neither ligand-gated nor voltage-gated.
B. The default state of the K+ leak channel is open.
C. The K+ leak channel exists throughout all 4 zones of the neuronal membrane.
D. The K+ leak channel results in a high resting permeability to K+. This is a major factor in determining the RMP.
diffusional force of K+
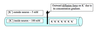
Membrane Potential’s affect on K+
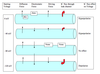
What is driving force?
The driving force determines the rate of flux. It is the sum of the diffusion and electrostatic forces. Driving force is calculated by Vm - Eion.
What is diffusion force?
Diffusion force is the force on an ion due to its concentration gradient (i.e. the ratio of extracellular to intracellular concentration of an ion). Does not change significantly under physiological conditions.
What is electrostatic force?
Electrostatic force is the force on an ion produced by the membrane voltage. The amount and direction of the force is a function of the membrane voltage and the charge of the ion.
What is driving force?
Driving force on an ion is the sum of the diffusion and electrostatic forces. It is calculated by the formula Vm - Eion (membrane voltage minus ionic Eion). The driving force is the force that controls the rate of ion flux IF the membrane is permeable to the ion. The ion flux thus alters the Vm. The direction of driving force tells you the direction of current flow: outward is positive, inward is negative.
What is ion flux?
Ion flux is the mechanism by which membrane potential is changed. It is controlled by 1) the driving force of an ion, and 2) the permeability of the membrane to that ion.
What is equilibrium potential?
Equilibrium potential (Eion) is a voltage that exactly offsets the diffusional force of the ion. It is the point where diffusion and electrostatic forces counteract each other. It is calculated by the Nernst Equation.
IMPORTANT: The membrane potential is always driven toward the Eion of the ion to which the membrane is most permeable.
Table of ion concentrations

Nernst Equation
Eion = 2.303 RT/zF log [ion]out/[ion]in
R = gas constant
T = absolute temperature
z = valence of the ion (charge) (i.e. + 1 for K+; F = Faraday’s constant.
+ 2 for Ca++ )
At body temperature of 37 degrees C, the Nernst equation for potassium simplifies to…
2
EK = 61.54 mV log 5/100 = 61.54 mV log (0.05) = 61.54 mV (-1.3)
= - 80mV
Below is a simplification of the first part of the Nernst Equation for key ions:
2.303 RT/zF = 61.54 mV for Na+ and K+
= - 61.54 mV for Cl-
= 61.54/2 mV (i.e., 30.77 mV) for Ca2+
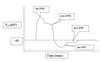
What is an action potential?
An action potential is an explosive depolarization of membrane potential (from -55 mV up to +40 mV - toward ENa).