Honors - Unit 4 - Chemical Quantities Flashcards
Empirical Formula
Empirical Formula - A formula that gives the simplest whole-number ratio of atoms in a compound.
Dimensional Analysis
A method of mathmatical analysis which involves converting between units.

Percent Composition
The amount of a given element in a compound.

Scientific Notation
A way of expressing really large or really small numbers.
Negative Exponents in Scientific Notation
Negative Exponents in Scientific Notation mean you should move the decimal to the left to change the number to standard notation.

Turning Standard Notation into Scientific Notation
If the number is greater than 1, the exponent will be positive.
If the number is less than 1, the exponent will be negative.

The Mole
A unit of measure.
1 mole = the atomic mass of an element
and
1 mole = 6.02 E23 atoms, particles, molecules, units, etc.
Positive Exponents in Scientific Notation
Postive Exponents mean move the decimal to the right when changing a number to standard notation.
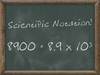
Significant Figures
All the digits in a measurement that are known with certainty plus a last digit that must be estimated.

Rules for Multiplying and Dividing with Significant Figures
The result has the same number of significant figures as the factor with the fewest significant figures.

Rules for Adding and Subtracting with Significant Figures
The result has the same number of decimal places as the number with the fewest decimal places.

How many atoms are in 2.7 moles of iron?
2.7 moles Fe x (6.02x10²³ atoms Fe/1 mole Fe) =
1.6 x 10²⁴atoms Fe
How many moles are in 3.67 x 10²⁴ molecules of SO₂?
- 67 x 10²⁴ molecules SO₂ x (1 mole SO₂/6.02x10²³ molecules SO₂)=
- 10 moles SO₂
9.08 g of Al₂0₃ is equal to how many moles?
9.08 g Al₂0₃ x (1 mole Al₂0₃/101.96 g Al₂0₃) = 0.0890 moles Al₂0₃
What mass of copper (II) sulfide is equal to 4.79 x 10²² atoms?
- 79 x 10²² atoms CuS x (1 mole CuS/6.02x10²³ atoms CuS) x (95.61 g CuS/1 mole CuS) =
- 61 g CuS
What is the percent composition of oxygen in CuSO₄?
mass of CuSO₄= 63.546 + 32.065 + 4(15.999) = 159.7 grams
Mass of oxygen = 4(15.999) = 64 grams
(64/159.7) x (100%) = 40.1. %O
A compound contains 21.6 g of silver and 3.21 g of sulfur, what is the percent composition?
21.6/(21.6+3.21) = 87.1% Ag
3.21/(21.6+3.21) = 12.9%S
Scientific Notation Example
Scientific notation (exponential notation) represents very large or very small numbers as powers of ten.

To convert a number greater than 1 to scientific notation?
The original decimal point is moved n places to the left, and the resulting number is multiplied by 10^n.

To convert a number less than 1 to scientific notation?
the original decimal point is moved n places to the right, and the resulting number is multiplied by 10^-n.

Significant Figure Rules for Addition and Subtraction:?
The answer of a calculation based on measurements cannot have greater significance than any of the measurements that produced the answer.
The least certain measurement (fewest decimal places) limits the certainty of the calculated answer.
*Line up the decimal points and report the answer based upon the fewest decimal places.
Significant Figure Rules for Multiplication and Division:?
The calculated answer can be no more precise than the least precise number from which the answer is derived.
The least precise number is the one with the fewest sig figs.

Rules for Rounding Off Numbers?
- When the leftmost number removed is less than 5, the preceding number is left unchanged.
Ex: Round 45.2624 to four significant digits: 45.26
- When the leftmost digit removed is 5 or greater, the
preceding number is increased by 1.
Ex: Round 3.75 to two significant figures: 3.8
Rules for Significant Figures?
1) ALL non-zero numbers (1,2,3,4,5,6,7,8,9) are ALWAYS significant.
2) LEADING zeroes are NEVER significant
3) Zeroes CAPTURED between non-zero numbers are ALWAYS significant.
4) TRAILING zeroes without a decimal are NEVER significant
5) TRAILING zeroes with a decimal are ALWAYS significant.
6) When using SCIENTIFIC NOTATION all digits in front of “x 10‾³” or “E-3” are ALWAYS significant
Subscripts
Small numbers to the lower right of chemical symbols. Represent the number of atoms of each element in a molecule.
0₂
Coefficients
The large numbers in front of chemical formulas. Represent the number of molecules of the substance in the reaction.
2Cl
Diatomic Molecule
If these elements appear by themselves in an equation, the must be in pairs with the subscript 2.
Scientific Notation in TI-84 and class calculators
(Number) 2nd EE (Exponent)
Hint: negative button is not the minus button
What do significant figures show?
Precision
Are digits 1-9 “significant” or “not significant”?
Significant
Are zeros between significant digits “significant” or “not significant”?
Significant
Are zeros before significant digits “significant” or “not significant”?
Not Significant
Are zeros after a decimal and after a significant digit “significant” or “not significant”?
Significant
When adding and subtracting with Significant Figures, ___________________.
keep the least number of decimal places
When multiplying and dividing with Significant Figures, ___________________.
round to least number of sig figs
Are zeros after a decimal and before a significant digit “significant” or “not significant”?
Not Significant
Convert the following into standard notation:
4.386 x 10⁷
43,860,000
Convert the following into standard notation:
4.39 E-7
0.000000439
Convert the following into standard notation:
3.7 x 10⁻⁵
0.000037
Steps for finding Empirical Formula from percent composition





