Environmental Pathogens Flashcards
IN a normal person responsible for allergies, Immunocompromised individuals who go outside creating a dangerous infection for indivduals like this. also takes part in Tb function
Aspergillus fumigatus
Pathogen category: mold
Source: environmentalreservoirs
Virulence factors: cell wall, septatebranching hyphae
Clinical: allergic asthma,aspergilloma, invasive
Immune response: cell-mediated immunity
Treatment and/or prevention:surgicaldebridement of fungal balls; voriconazole
Aspergillusfumigatus(and other species) are common environmental molds.
Everyone exposed, fought off by macrophages creating allergy affect
Aspergillus fumigatushas an impact on humans in three major ways:
- IgE-mediated allergic response to the spores with inhalation ex. wheezing
- Aspergillomaor a fungal ball in a preexisting lung cavity, as from TB
- Invasiveaspergillosisin an immunocompromised patient
Givenpredospositionto this bug if immunecompromised
Caspofungin- similar to daptomycinusing long fatty acid tail to anchor itself into the membrane and more complicated ring interferes w/ its B-glucan synthesis (important sugars that keep lay basis of anchoring for chitin (chitin- KOH prep keeping fungi visible) can also be used for candida not responding
amphtoericinB-not effectivein 10 years standardstrategy for severe fungal infections targettingergosterolw/in cell membrane
If disseminated, Aspergillus can kill quickly, given its predilection to penetrating endothelia, as seen above. Whilethe more recent azole voriconazoleis typically effective, the echinocandinsare (expensive) alternatives for disseminated Aspergillus, and are primary indications for disseminated candidiasis as well. The echinocandinsinhibit the fungal cell wall by inhibiting the synthesis of beta glucans that cross link other cell wall components (the long fatty acid tail helps to anchor the molecule in the fungal cell membrane to “keep it in place” while inhibiting the glucans).
daibeteic patient comes in w/ a cellulitis that can turn into a deadly nose and eye infection. grows on bread
Mucorand Rhizopusspecies
Pathogen category: molds
Source: environment; breathing in of spores
Virulence factors: fungal cell wall with chitin; ketone reductase tofunction in diabetic ketogenic environment
Clinical: mucormycosis/zygomycosis
Immune response: sporesnormally cleared by physical means, e.g., mucociliarytransport or by phagocytes
Treatment and/or prevention:diabetic control; surgical debridement and amphotericin B
Goes on bead in fridge, no septa on this bug and can break up. Not well controlled diabetes (ketotic-acidotic) hyperglycemic neutrophil not working well have a ketone reductasefavoring these indivdualsand using them as food source (rhinocerebralmucor) early cellulitis type picture.
Must debride contamintatedtissue and high amphotericin B
Mucoror Rhizopusspecies are environmental molds. In contrast to Aspergillus, which hashyphae that branch at 45odegree angle, Mucorbranches at right angles and as this image shows, is aseptate(without partitions). This lack of partitioning can make aclinical sample break apart, such as the fragment seen here.Mucoror Rhizopusspecies are most likely seen in individuals with leukemia, diabetes mellitus, or other immunocompromising condition. Poorlycontrolled diabetic patients are most at risk, with a combination of hyperglycemia, impaired neutrophil function, and the fungus with the ability to handle ketoacidoticsituations with a ketone reductase enzyme. The spores are breathed in and hyphaesubsequently generate and proliferate.
The most common presentation is rhinocerebralmucormycosis,which can be lethal. Surgicaldebridement in addition to high-dose amphotericin B would be appropriate.
What are factors that make an individual immunocompromsied?

What are common causes of compromised immunity and their consequences?
Break in skin: bacteroides(peritonitis) , s. aureus
Normal fleura: C. diff, lactobacillus (vaginal secondary yeast)
Born w/ dysfunction of neutrophils due to low count such as neutropenia (flourishing bacteria where you wouldn’t expect S. aureus) hyperglycemia affecting function of neutrophils (not working as well developing fungal infection
Humoral function: no IgGfor opsonizing or IgA (may cause respiratory infect or pyogenic opsonziedand taken care of)
Lack of spleen- septicemia organisms normally targettedby macrophages
Neisseria- avoiding complement or if missing C3 (not first go through of staph and strep pyogenic)
Cell-mediated- anythingat risk no stimulation of cytotoxic T cells

What is zoonosis? what are vecotr borne illnesses? Zoonosis: animal disease transmissible to humanbeings
Zoonosis: animal disease transmissible to humanbeings
vector-borne illnesses and zoonoses- animal reservoir from anialspread usually tick or mosquito carrier
What are the different types of transmission of zoonosis?

Respiratory, gastrointestinal, and sexual pathogens in the upper row have been previously covered.
Sandflyfevere,
Brucellosis, rabies, slmonlelosis
Plague, trypanosomiasus (sleeping sickness) yellow fever
What are the emerging diseases? zoonoses, potentail agents of bioterrorism which are similar or different?
Anotherconsideration, given where we are in 2019, is that a number of the diseases covered this weekcan be considered possible bioterrorism agents.
Viralhemorhagicfever- ebolaor hiking rodent borne viruses
Lyme disease, avian influenze, west nile, E. coli (EHEC)

What are the specialty features and functions of spirochetes?
Asseen with syphilis, spirochetes are very thin Gram-negative organisms with tight coils around an axial filament offlagella in the periplasmic space that allows for a “corkscrew” penetration of tissues.
Stretched out, outer sheet and membrane buriedin periplasm, cell membrane surrounding cytoplasm. Good at coiling, digging, and penetrating given strctureusing periplasmicflagella driving through tissues.
Using membrane can be off the pathogen screen can prevent same immunologic profile, w/ shifting population of outer surface protein making a diffeentimmunologic profile depending upon its stage in its life cycle

What is this disease?
How is it spread?
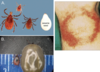
Borrelia burgdorferi: Lyme diseaseis spread by deer tick(genus Ixodes) bites. The target skin lesion of erythemamigransshown should be present in a majority of patients. Doxycycline as first level antibiotic, with considerations to ceftriaxone if additional complications, e.g., neuropathies or cardiovascular, occur.
Borreliaburgdorferi
Pathogen category: spirochete
Source: tick-borne(Ixodes) from white-foot mouse and whitetail deer populations
Virulence factors: surface antigenicvariation, use of host proteins like plasmin to “dissolve” through tissues
Clinical: Lymedisease
Immune response: Igs, CTLsgenerated, but surface antigenic variation
Treatment and/or prevention:tick avoidanceand early removal; doxycycline, with ceftriaxonefor more severe disease
Deersupport the tick, while white-footed mice are the main reservoir. DEET insect repellant and tick removal are first lines of defense; a vaccine directed against the organism’s OspA(to kill the Borreliain the tick gut before they expressed OspC) has been discontinued from the US market since 2002.
The Northeast and mid-Atlantic statesare the high-risk regions for Lyme disease. Outbreak of children in 70s from rheumatoid arthritis found it was lymedisase.

What is the life cycle and stages of lyme disease?
Borrelia burgdorferi: Lyme diseaseis spread by deer tick(genus Ixodes) bites. The target skin lesion of eryethemamigransshown should be present in a majority of patients,but diagnosis is typically done by serology or PCR.
As with the other spirochete we studied so far, syphilis, there are three general stages to the infection.
stage 1: the erythema migransin ~80% of patients, with flu-like symptoms in ~ 50%. The tick will have needed to feed for 24 or more hours to make transmission likely. Bacterial numbers multiply in the tick with feeding, and it will take some time for them to migrate to the salivary glands. Expression of Osp(outer surface protein) affects the transmission from the tick to the human host. Activation from one Ospclass (OspA-multiplying in the tick gut) to another (OspC-upregulatedto live in humans) takes time and accounts for some of this delay. In addition, bacterial numbers multiply in the tick with feeding, and this will take some time to migrate to the salivary glands. Needs to feed forseveral days to build up and multiply to pass
stage2:( (similar to syphilis) weeks to months later (Borelliais slow-growing) may be the pattern of neurologic, e.g., Bell palsy following penetration of the blood-brain barrier, and cardiovascular, e.g., AV block, following hematogenousspread. The spirochete does not generate proteases to move through the extracellularmatrix, but binds human ones to help with that progression. In addition, Borreliawill undergo some surface protein downregulation (e.g., of OspC) and surface antigen variation in order to help evade immune responses.Human extracellular matrixproteins as it is transmitted through human can pick up plasmids and take advantage of surface features to protect it- doesn’t do it that much
stage 3: months to years later may appear polyarthritis or chronic neurologic deficits, e.g., cognitive and mood disturbances, may appear as a later finding, presumably triggered by immunologic response to the ongoing infection. Brain, chronic changesto the skin, arthritis
Vaccine:- antibodies made against this and would create IgGto try to prevent lymefrom getting effective in stage 1. Vaccine was not 100% protective, couldn’t use the testing process because always appeared positive. Could not protect against stage 2- even strong immune response can still get through
Need antibiotic w/ high ability to get through good tissue penetration
Doxycycline and ceftriaxone- if really bad
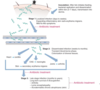
What is chronic lye/post-treatment lyme disease syndrome? how do you treat it?
treatment above and beyond of just doxy and ceftriazone have not shown ability to treat chronic symptoms for extended periods
due to hidden material that macrophages are not able to clear it w/ little chunks of DNA and cytoplasmic remnants can getting them activated even after the infection is gone. burrelia leave chunks of peptidoglycan triggering ongoing state of infection w/out activating it (cell wall hiding in periplasm- stays off of immunologic membrane)
chronic Lyme disease, more precisely definedas post-treatment Lyme disease syndrome(PTLDS): an operational definition proposed by the Infectious Disease Society of America in orderto identify and study those with early Lyme disease that is treated, but continue to have pain, fatigue, and cognitive complaints that impact everyday activities (~10% of patients with Lyme). There has been no widely accepted evidence of spirochete persistence following adequate initial treatment (ISDA guidelines of up to 30 days), and no consistently demonstrated benefit to chronic high-dose regimens for months to a year.
model of bacterial DNA persistence triggering an ongoing innate system activation even after viable bacteria have been cleared by antibiotics with good tissue penetration.

What are the ricketsias? what pathogens are in this class?
We will look at tick-borne examples ofrickettsias(-ae) as well.
Ricketsia: anaplasmatacease.
Therickettsiasare obligate intracellular parasites (tick or human for life cycle) that do not make much ATP. They are usually spread by an arthropod vector.
Doxycyclineis the current drug of choice for the rickettsialdiseases (good tissue penetration given its lipophilic nature and its bacteriostaticproperties allow the immune system to “catch up” to clear the rickettsia).
The genus Wolbachiaunderneath the Anaplasmain the cladogram above is an endosymbiont with filarial worms such as Onchocerca.

describe the ricketsia pathogenesis?
Enter, enter epithliathen escape from immune system and inject itself into nearby cells avoiding complement and antibodies. Once that cell breaks open can get necrosis and requires a strong cell-mediated response to clear endothelium that regenerates. Will develop a very strong rash
The intracellularpattern and need for cell-mediated immunity to generate clearance of rickettsiaeare apparent in these images. Note how they can propel from cell to cell quickly via actin polymerization (similar to the strategy seen with Listeria), and so if untreated, Rickettsiarickettsiican infect a lot of endothelium rapidly.
Can be lethal for kids and seniors will also affect things like the liver. Bite site develops necrosis and will develop scarring
Al responsive to doxycylcine

petechial rash from being breached by necrosis progress on limbs and progresses centrally vasculitis. fever, rash, and tick exposure common in the southeast US
Rickettsiarickettsii
Pathogen category: Gram-negative, aerobic obligate intracellular coccobacillus
Source: ticks (Dermacentorspecies)
Virulence factors: abilityto escape the phagosome and remain intracellular; ability to derive ATP and other materials from host cell
Clinical: RockyMountain spotted fever
Immune response: strong cell-mediated response to clear infected endothelial cells
Treatment and/or prevention:tick avoidance;doxycycline (chloramphenicol as alternative in pregnancy)
Rocky Mountain spotted fever(RMSF) is tick-spread, by members of the genus Dermacentor(wood or dog tick), and as the map indicates.Not located in rocky mountain but the south.
Anendothelial invasionhelping to account for the vasculiticrash. Abrupt onset of high fever and chills precedes the rash. Given high levels of lethality, prompt presumptive treatment is important, as a combinationof fever, rash, and tick exposure is classic, but present in < 20%. The rash tends to start in the limbs and progresscentrally.
While RMSF and R.rickettsiiis the main pathogen, CDC recognizes R. parkeriand similar diseases as “spottedfever rickettsiosis (SFRs)” along with RMSF.
Cease ongoing multiplactionof ongoing pathogen- doxyclylinefirst line agent for most ricketsiagood tissue pentrationand kids (due to potential bone impacts)
fver commonly found in texas
Otherrickettsias, such as Ehrlichiachaffeenis, transmitted by the Lone Star tick (from a deer reservoir), presents with a systemic illness of human monocyticehrlichiosis;
As they are preferentially found in leukocytes, these infections will not present as with much rash as RMSF (rash is rare in HGA), but both HME and HGA will generate fever, leukocytopenia, and thrombocytopenia (presumably from activated immune cells). Developing interluekin1, TNFdeveloping fever pattern.
Ehrlichiachaffeenis- lone star tick cluster of monocytes
Pathogen category: rickettsia(obligate aerobicintracellular Gram-negative coccobacillus)
Source: ticks (genus Amblyomma) from differentanimal reservoirs, e.g., deer
Virulence factors: ability to persistin macrophages
Clinical: human monocyticehrlichiosis(MHE)
Immune response: TNF-alpha overproduction by CD8 cells
Treatment and/or prevention:tick avoidance or prompt removal;doxycycline

fever commonly found tick borne illness in rennseallaer county
Anaplasmaphagocytophilum, transmitted by the deer or western black-legged tick, causes human granulocytic anaplasmosis. Tic born infections NEED BLOOD SMEAR
As they are preferentially found in leukocytes, these infections will not present as with much rash as RMSF (rash is rare in HGA), but both HME and HGA will generate fever, leukocytopenia, and thrombocytopenia (presumably from activated immune cells). Developing interluekin1, TNFdeveloping fever pattern.
Anaplasmaphagocytophilum-granulomatuscells of neutrophils
Pathogen category: rickettsia(obligate aerobicintracellular Gram-negative coccobacillus)
Source: ticks (genus Ixodes) from differentanimal reservoirs, e.g., deer, white-foot mouse
Virulence factors: inhibitsneutrophilapoptosis
Clinical: human granulocytic anaplasmosis(HGA)
Immune response: Th1-stimulatedmacrophage clearance
Treatment and/or prevention:tick avoidance or prompt removal;doxycycline

Q fever(acute febrile illness to atypical pneumonia)
Coxiellaburnetii
Pathogen category: rickettsia(obligate aerobicintracellular Gram-negative coccobacillus)
Source: farm animalsproducts, e.g., aerosolized wastes
Virulence factors: environmentallyhardy organisms resistant to drying
Clinical: Q fever(acute febrile illness to atypical pneumonia)
Immune response: Igs, with cell-mediatedimmunity to help clear the infected phagocytes
Treatment and/or prevention:doxycycline
Coxiellaburnetii: unlike other rickettsiae, there is no arthropodvector, as it is spread by inhalationfrom domestic livestock wastes, e.g., afterbirth materials, and will persist in macrophagesas shown above (Coxiellais actually related more to Legionellaand Francisella, which also persist in phagocytic cells). Diagnose via serology. Q feverincludes fever and pneumonia without rash. A resistance to drying makes Coxiellaa possible bioterrorismagent(due to its ability to sporulateform breathing in)
Behaveslike ricketisa(legionaresdisease- taken up by phagocytic cells) similar in function to legionares. Structurally similar to ricketsia. Animal infection (farm animals-sheep and cattle)- respiratory infection because of breathing in feces (alveolar macrophages) when trying to kill intracellular pathogen TNF and IL1.

flea bites develop swollen lymph nodes

Yersiniapestis
Pathogen category: Gram-negative rod; aerobic,intracellular
Source: flea bites from animal reservoirs such as rats andprairie dogs
Virulence factors: coagulase; outer membrane proteins
Clinical: bubonicplague
Immune response: inflammatory responseby neutrophils; cell-mediated immunity to clear infected cells; Igsformed
Treatment and/or prevention:reservoir(and hence flea) avoidance; gentamicin (since it is very difficult to kill)
Yersinia pestis: Gram negative rod responsible for bubonic plague. The pneumonic form of plague has a very high mortality rate, even with antibiotics such as gentamicin as the current drug of choice, with fluoroquinolones or doxycycline as alternatives. Dryer parts of west coast requiring: rats, wild rodents (prairiedogs).
If someone if bit fought off at local lymph node (similar to chlamydia and cat scratch fever), hematomogicspread can develop deadly respiratory infection.
Theflea virulence factors trigger multiplication of the bacteria and clotting off of the gut, so that the starving flea keeps biting. The Yersiniaouter membrane proteins trigger cell destruction once injected. (often on the limbs, legs, hairline)
As with Y.enterocolitica, Y. pestismultiplies in the local lymph nodes, with plague associatedwith the enlarged and painfully swollen bubo(local lymph node battle similar to yersinia). Coagulation factors produced by the tick in order to satiate it. Produce exotoxin (type III secretion) if not fighting off and having initatlinnate at site of infection and become big and proliferative (bubo).
In addition, plague can generate a F1 proteinto create a capsule-like (allow for hematogonicspread), antiphagocyticeffect, and so lead to a bacteremia. This bacteremiawith a Gram-negative organism with lipopolysaccharidecan trigger septic pattern, e.g., with DIC and purpuracreating a Black Death-type pattern.
loss of blood vessel tone developing black death pattern.

person runs over a warren den and developed local infection of fingers
Francisellatularensis
Pathogen category: Gram-negativerod, aerobic, intracellular
Source: multiplewild mammals reservoirs, e.g., muskrats, rabbits; with tick/deer fly/mosquito vectors
Virulence factors: very low numbers needed for infection; phagolysomalescape
Clinical: tularemia
Immune response: strong cell-mediated response to activate phagocytes to clear the intracellular pathogens
Treatment and/or prevention:avoidance of bite; aminoglycoside (want to kill since it takes so few to infect)
Francisellatularensis: tularemia:Gram negative rod susceptible to the aminoglycosides gentamicin, tobramycin, or streptomycin, although like plague, it can be covered with doxycycline if mild. Albany Med is a major tularemia research center.
Spread by mosquitoes, bunnies,muskrats through direct contact, ingestion, bite. Intracellular pathogen takes very few pathogens for large establishment (wide-spread ill defined infection, or localized necrosis) . If have strong cell-mediated response can stay localized (if big infectious response from large dose of it can cause high level of infection
“Rabbit fever” spread by abrasion or inhalation, with fever, enlarged nodes, and ulceration at the site of infection. There may be a systemic typhoidaldisease with high levels of mortality. Three possibilities of infection:
- tick bite
- infectious aerosol from skinning infected animal
- consumption of infected meats
If internalized,Francisellamay trigger a granulomatous response to try to clear it.

sewage worker develops jaundice and hemorrhagic conjunctivits
Leptospirainterrogans
Pathogen category: spirochete
Source: mammal renal reservoir,particularly rats
Virulence factors: persistence in wet soil or water,with transmission through breaks in skin/mucosae; burrow through tissues and systemic spread as with other spirochetal diseases
Clinical: leptospirosis; Weil disease as most severe form with hemorrhage combinedwith renal and hepatic failure
Immune response: antibodiesgenerated; vasculitis pattern generated with the tissue penetration
Treatment and/or prevention:avoidinfected water; disease often asymptomatic; mild: doxycycline; severe: penicillin G or ceftriaxone
Leptospirais a common spirochete zoonosis that seta up a chronic renal infection in animals, such that exposure to rat urine can be a major risk factor. Like other spirochetes, it can penetrate through tissue to then be spread lymphatically and hematologicallythroughout. Given a vasculitis-type targeting, it is not a surprise that there can be hemorrhagic components to severe disease.
Targets renal tissue-urine (damp soileexposure, rats and dogs normal carriers), sewage workers or product, most are mild unpleasantness, can behave like spirochete penetrating multiple tissues.
Severe illness or weil’sdisease- hemorrhagic confunctivits, jaundice.

spread to humans via direct contact through broken skin or via ingestion of contaminated products. A prolonged infection can follow a month-long incubation, e.g., with fever, aches, and weakness. There can be chronic disease if it is not cleared.

Brucellaisanother common world-wide pathogen that can be spread to humans via direct contact through broken skin or via ingestion of contaminated products. A prolonged infection can follow a month-long incubation, e.g., with fever, aches, and weakness. There can be chronic disease if it is not cleared.
Intracellular, smoldering infection, granulomatous, antibiotic resistance,
Brucella species(B. melitensisas main one)
Pathogen category: intracellularGram-negative rod
Source: mammal reservoirs
Virulence factors: intracellular persistence
Clinical: brucellosis (undulant fever)
Immune response: lymphatic spread with infectionof macrophages in reticuloendothelial tissue and subsequent granuloma formation. Antibodies generated but not effective.
Treatment and/or prevention:avoidanceof raw dairy products or infected animal materials; doxycycline + gentamycin
What are some examples of vetor borne parasites? update w/ new picture?

paitent lives in africa has recurrent spikes of fever. in children develops stroke like illness
Asan overviewof the overall malarialife cycle, from sporozoiteinjected by the female Anophelesmosquito, to merozoitesreleased by the liver, to trophozoitesfeeding in the red cells, to gametocytes taken up by the mosquito, that form a zygote, which develops into an oocyst, which then forms sporozoites….
Malaria paraistemany different viewpoints w/ which to infect
1) Suck up male female gametocyte forms (in the salivary and stomach of mosquito). First form injected sporozoites,
2) predilection to liver tissue and strains of malaria can hang out all the time (hypnozoites).
3) Merosozoite- Porliferateto red cells full of hemoglobin (longest exposure and adding antibody most chance of being knocked out)
4) TrophozoiteLead to feeding stages
5) Reproduce againt
Plasmodium species
Pathogen category: sporozoanparasite(apicomplexan)
Source: mosquitos (Anophelesspecies)
Virulence factors: variationin merozoitesurface proteins; minimal extracellular exposure as rapid uptake into erythrocytes
Clinical: malaria
Immune response: merozoiterelease triggers macrophage production of TNF-alpha, IL-1; Igsgenerated but not likely totally protective (given the surface variation of merozoites)
Treatment and/or prevention:mosquitopopulation control; mosquito bite avoidance; chemoprophylaxis, e.g., doxycyline; treatment with proguanil-atoquavone(Malarone) or quinine-based products;

What is the life cycle and responses
The repeating fevers represent the immune response to the merozoitesbursting out of the red cells. It is not a surprise that given the different stages of malaria, there are different immune responses along the way. The generation of antibodies for the blood-borne stages is the basis for vaccine development, particularly with the blood-borne sporozoite stages before liver involvement (the basis for the currently approved vaccine against P. falciparum with < 50% efficacy, which is currently being piloted as part of routine child vaccinations by the WHO, which started in 2018, in Ghana, Kenya, and Malawi).
Recurrent spikes of fever most cycle every 2 days













