Clinical Trials Flashcards
What is a clinical trial?
Any form of planned experiment which involves patients and is designed to elicidate the most appropriate method of treatment for future patients with a medical condition
What are the 2 main purposes of a clinical trial?
To provide reliable evidence of treatment efficacy & safety
Efficacy: ability of intervention to improve the health of a defined group under specific conditions
Safety: ability of intervention not to harm a defined group under specific conditions
What 3 things must a clinical trial be in order to give a fair comparison of effect and safety?
- Reproducible - in experiemental conditions
- Controlled - comparison of interventions to show it is the intervention that’s made the difference
- Fair - unbiased without confounding
What is the difference between efficacy and effectiveness?
Efficacy = ideal clinical conditions
Effectiveness = real world clinical experience
What are the 4 different phases of a clinical trial?

What are non-randomised clinical trials?
Clinical trials involving the allocation of patients recieving a new treatment to compare with a group of patients recieving the standard treatment
What are the disadvantages of non-randomised clinical trials?
Allocation bias - by patient, clinician or investigator
Confounding - known and unknown
What are comparison with historical controls?
Comparison of a group of patients who had the standard treatment with a group of patients recieving new treatment
What are the disadvantages of using comparison of historical controls for the ‘standard treatment’ group?
- Selection less rigorous
- treated differently from ‘new treatment’ group
- less information about bias/ confounders
- unable to control for confounders
Is this observed rate ratio statistically significant? Explain why
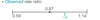
NO
The observed rate ratio and confidence interval crosses 1 therefore an affect may be due to chance
1= no effect at all
For what reasons do you need to pre-define trial outcomes at the start of the trial?
- prevent ‘data dredgind’, ‘repeated analyses’
- protocol for data collection
- agree criteris for measurement and assessment of outcomes
What is the difference between primary and secondary outcomes?
Primary Outcomes:
- Only one primary outcome
- Used in the sample size calculation
Secondary Outcomes:
- other outcomes of interest
- often includens occurence of side effects
What are the 3 different types of trial outcome?
Patho-physiological
- tumour size, thyroixine levels, ECG changes
Clinically defined
- death (mortality)
- disease (morbidity)
- disability
Patient-focused
- QoL
- psychological well-defined
- social well being
- satisfaction
What are the features of an ideal trial outcome?
- Appropriate and Relevant
- Valid and attributable (linked to the treatment compared)
- Sensitive and Specific
- Reliable and Robust
- Simple and Sustainable
- Cheap and Timely
How are measurements timed throughout a clinical trial?
- Baseline measurement taken of relevant factors
- Monitoring during the trial
- for possible effect
- for adverse effects
- Final measurement of outcomes
What are the benefits of random allocation in a trial?
-
Minimal allocation bias
- Each participant has an equal chance of being allocated to the treatments in the trial
-
Minimal confounding
- treatment groups likely to be similar in size and characteristics by chance
What is the best way to randomise a trial?
Use an external unit to create a computer generated random number that is accessed remotely
What are the disadvantages of open label trials (knowing the treatment allocation)?
- Patient may alter behaviour or expectations of outcome (behaviour effect)
- Clinician may alter their treatment, care and interest in patient (non-treatment effect)
- Investigator may alter their approach when making measurements and assessing outcomes (measurement bias)
What are the different types of blinding of a trial?
Single-blind - one of; patient, clinician or assessor does not know treatment
Double-blind - two of; patient, clinican or assessor does not know the treatment allocation
Triple blind - implies all do not know the allocation
Give examples of when blinding a trial may be difficult?
- Surgical procedures
- Pychotherapy vs anti-depressants
- Using alternative medicine
- Lifestyle interventions
- Prevention programmes
What is the placebo effect?
Even if the therapy is irrelevant to the patients condition, the patient’s attitude to their illness or the illness itself may be improved by a feeling that something is being done about it
What is a placebo?
An inert substance made to appear identical in every way to the active formulation with which it is to be compared
Explain what it means that active treatment has placebo in it?
Giving any kind of treatment has placebo effect
By comparing with a placebo, it cancels out the placebo effect that exists within active treatment

What are some of the ethical implications of placebo?
- should only be used when no standard treatment is available
- Use of placebo is a form of deception
- Essential all participants are informed that they may recieve a placebo
What is meant by losses to follow up?
Not every participant will remain in the trial
- Their clinical condition may be necessitate their removal from the trial (appropriate)
- They may choose to withdraw from trial (unfortunate)
How can you minimise losses to follow up?
- Make follow up practical and minimise inconvenience
- Be honest about commitment required from participants
- Avoid Coercion
- Maintain contact with paticipants
Why may patients not comply with their allocated treatment?
- Mis-understood instructions
- May not like taking their treatment
- May think it’s not working
- May prefer other treatments
- Can’t be bothered
How can you maximise compliance in a trial?
- Simplify instructions
- Ask about compliance
- Ask about effects/ side effects
- Monitor compliance; tablet count, urine level, blood level
What is ‘as treated analysis’
Only the patients who have taken their treatment allocation as indicated are measured in final analysis
Only interested in the physiological effect of drug on body
What is ‘intention-to-treat’ analysis?
Everyone is analysed, regardless of whether they took the drug or not
Reflects real life practice
What are the disadvantages of ‘as-treated’ analysis?
- Loses the effect of randomisation
- Non compliers likely to be systemically different from compliers
- Introduced selection bias and confounding
What is the main benefit of ‘intention to treat’ analysis?
Preserves the effect of randomisation
Minimal selection bias and confounding
What is the difference between collective and individual ethics?
Collective:
- All patients should ahve treatments that are properly tested for efficacy and safety
Individual:
- Priniciple of beneficence
- principle of non-maleficence
- principle of autonomy
- principle of justice
What is clinical equipoise?
When there is reasonable uncertainty or gernuine ignorance about the better treatemtn of intervention (including non-intervention)
What are the roles of reseatch ethics committee?
Focus on:
- Scientific design and conduct of the study
- Recruitment of research participants
- Care and protection of research participants
- Protction of research participant’s confidentiality
- Informed consent process
- Community considerations


