Chemistry/Physiology Recap Flashcards
Chemical energy
- Stored in bonds of chemical substances
- ex. Glucose (C6H12O6)
The energy is stored in the covalent bonds between carbon atoms

Some bacteria require energy in the form of the _______________.These bacteria cannot manufacture food like ___________ nor absorb nutrients from the environment like _________.
- Organic Molecules
- photosynthetic organisms
- fungi
The organic ___________ some bacteria take up are broken down to make _______.
- molecules
- ATP
________ provides the energy cells need to stay alive.
ATP (Adenosine Triphosphate)
_________ in molecules is stored in the ___________between _________ that make up the molecules.
- energy
- bonds
- carbon atoms
definition of chemistry
Chemistry is the study of interactions between atoms and molecules
definition of atom
The atom is the smallest unit of matter and cannot be subdivided into smaller substances
Atoms interact to form ___________.
molecules
Atoms are composed of ____________.
subatomic particles
what are the 3 subatomic particles?
Protons, neutrons, electrons
Protons
positively charged particles found in the nucleus of an atom.
Neutrons
neutrally charged particles found in the nucleus of an atom.
Electrons
negatively charged particles that orbit nucleus in an electron cloud
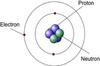
planetary model of an atom

orbital model
what are the 3 smallest elements?
- hydrogen
- helium
- lithium
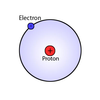
- hydrogen (H)
- 1 proton, 0 neutrons, 1 electron

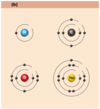
- helium atom (He)
- 2 protons, 2 neutrons, 2 electrons

- lithium atom (Li)
- 3 protons, 4 neutrons, 3 electrons
outer layer of e- is called the ________, and the e- in that layer are called __________.
- valence shell
- valence electrons
Only ___________are involved in ____________between any two atoms.
- valence electrons
- chemical bonding
what are the 4 chemically reactive elements?
- hydrogen
- carbon
- sodium
- oxygen
Most atoms chemically combined with other atoms to form __________________.
molecules and compounds
definition of a molecule
Two or more atoms bonded together (e.g., H2 or C6H12O6)
what is special about valence chemically reactive elements?
- Their valence shell is not full they tend to gain, lose, or share electrons (form bonds) with other atoms to achieve stability
Electrons in valence shell (outermost electron shell); __________________ and ______________
- Have most potential energy
- Are chemically reactive electrons
Octet rule (rule of eights)
Except for the first shell (full with two electrons) atoms interact to have eight electrons in their valence shell
single covalent bond
- one pair of electrons is shared between atoms
- ex. methane gas= CH4

double covalent bond
- two pairs of electrons are shared between atoms
- ex. oxygen gas O2

Triple covalent bond
- Three pairs of electrons are shared between atoms
- ex. Nitrogen gas

When two or more atoms join together to form molecules, the atoms form ____________.
- Chemical bonds that hold them togther
What are the 3 major types of chemical bonds?
- Covalent bonds
- Ionic bonds
- Hyrogen bonds
What are the 2 types of covalent bonds
- polar covalent bonds
- non-polar covalent bonds
Covalent Bonds
- Prefix “co” means “together”, as in cooperate (work together) or cohabitate (live together); “valent” refers to valence e- (in the outermost shell or layer).
- “covalent” means sharing e- pairs together in the valence shell. Keeping in mind the Octet Rule, in which the atoms are trying to make their valence shell “full”, one way to accomplish this is for two atoms to share e- pairs. If one atom shares one e- and the other atom shares another e-, then both atoms have the e- they want to complete their valence shell.
- Covalent bonds - Formed by sharing of two or more valence shell electrons such that each atom fills its valence shell at least part of the time
Non-polar covalent bond
- Electrons shared equally > nonpolar covalent bond
- Produces electrically balanced, nonpolar molecules such as CO2
- charge is balanced among atoms
- Carbon dioxide (CO2) molecules are linear and symmetrical. They are nonpolar

Polar Covalent bond
- Unequal sharing of electrons > polar covalent bond
- Atoms in bond have different electron-attracting abilities
- usually occurs with electronegative elements like oxygen or nitrogen.
- slight negative charge at one end of molecule and slight positive charge at the other end.
Electronegative
- oxygen and nitrogen are electronegative
- Strong electron-attracting ability
- Shares e- unequally with other atoms
- V-shaped water (H2O) molecules have two poles of charge—a slightly more negative oxygen end (d–) and a slightly more positive hydrogen end (d+).

Ionic bond
- complete transfer of electrons
- separate ions (charged particles) form
- ex. Na+ Cl-
- Transfer of valence shell electrons from one atom to another forms ions; Again, the transfer occurs so that both atoms fulfill the Octet Rule. It is easier for Na atoms to give up one e- than to gain 7 e-; similarly, it is easier for Cl to gain 1 e- than to lose 7 e-.
- One becomes an anion (negative charge), an atom that gained one or more electrons; One becomes a cation (positive charge), an atom that lost one or more electrons; Attraction of opposite charges results in an ionic bond

Hydrogen bonds
- Attractive force between hydrogen (usually δ+) of one molecule and an electronegative atom of another molecule or another atom in the same molecule
- Not true bond > no transfer or sharing of e- (but it is an attractive force between atoms)
- Hold individual water molecules together; gives water important properties for life (universal solvent, resistant to temp changes, cohesion and adhesion, evaporative cooling)
- H bonds also hold large molecules in their three-dimensional shape by acting as intramolecular bonds
- The slightly positive ends (d+) of the water molecules become aligned with the slightly negative ends (d–) of other water molecules.

Hydrogen bonds hold individual water molecules together (what kind of bond holds atoms together in a single water molecule?)
Polar covalent bonds
Hydrogen bonds gives water properties which are important for life, what are they?
- universal solvent
- resistant to temperature changes
- cohesion and adhesion
- evaporate cooling
Water is the universal solvent, what does that mean?
- it dissolves MANY substances
- water molecules surround the atoms of the substance being dissolved, forming “hydration spheres” around the atoms or parts of the molecules being dissolved. When forming hydration spheres around atoms, the partially negative O atoms in water molecules will point toward the positively charged atoms of the substance being dissolved (Na+ ions, in this case), and the partially positive H atoms will point toward the negatively charged atoms of the substance being dissolved (Cl- ion, in this case). In many cases, the substance is invisible once dissolved, but you still know it is present in the water (dissolved) because you can taste or smell it.

Chemical reactions
- Chemical reactions occur when chemical bonds are formed, rearranged, or broken.
- Represented as chemical equations using molecular formulas
- Chemical equations contain: Reactants (Number and kind of reacting substances), and Product(s)

Synthesis Reactions
- A + B —-> AB (e.g. 2H2 +O2 —-> 2H2O)
- Atoms or molecules combine to form larger, more complex molecule
- Always involve bond formation (Anabolic)
- reactants come togther to make a product
Decomposition Reactions
- AB ® A + B (e.g. NaCl à Na+ + Cl- )
- Molecule is broken down into smaller molecules or its constituent atoms
- Reverse of synthesis reactions
- Involve breaking of bonds (Catabolic)
- reactant is broken down into products
Exchange Reactions
- AB + C > AC + B (e.g. Zn + 2 HCl à H2 + ZnCl2 )
- Also called displacement reactions
- Involve both synthesis and decomposition
- Bonds are both made and broken
Rate
how quickly reactants are formed into products, regardless of the type of reaction.
The rate of chemical reactions are affected by:
- Temperature (increased temp, increased rate)
- Concentration of reactant (increase concentration, increased rate)
- Particle size (decreased particle size, increased rate.
- Catalysts increase Rate without being chemically changed or part of product
•Enzymes are biological catalysts
Chemical reactions are essential for all cells all the time!! Most do not occur naturally at a fast enough rate to provide products needed to stay alive. _____________ inside our cells are crucial for making the reactions occur fast enough to sustain life.
Enzymes
molecular weight
The sum of the atomic weights in a molecule is the molecular weight
One ________of a substance is its molecular weight in ___________.
- mole
- grams
- ex.
H2O
2H = 2 x 1 = 2 (2 hydrogen molecules times its molecular weight)
O = 16 (1 oxygen molecule’s molecular weight)
MW = 18 (moleclar weight of both)
1 mole weighs 18 g
cells contain about _______in the cytoplasm and are generally “bathed” in __________.Therefore all reactions are taking place in what we call an _______________ and for the most part all components of that reaction, including reactants, enzymes, and products, are _______ in water.
- 75% water
- watery fluid
- aqueous or watery enviornment
- dissolved
Solution
- Solvent (liquid) + solute(s) (dissolved particles), e.g. Kool Aid (water is solvent, Kool Aid powder and sugar are dissolved solutes)
- Solutions are often described in terms of their CONCENTRATION, or the amount of solute dissolved.
- In chemistry, this was sometimes referred to as molarity or molar concentration. The higher the molarity, the more solute per given volume of solvent
pH
is a measure of [hydrogen ion]
The pH scale ranges from _______.
0-14

a pH of 0-6.99 is _______.
acidic
a pH of 7.00 is ________.
neutral
a pH of 7.01-14.0 is _______.
basic
a pH scale is ___________. A pH 5 solution is ____times more acidic than a pH 6 solution and _______times more acidic than a pH 7 solution
- logarithmic
- 10
- 100
Another important aspect of the environment in which chemical reactions occur is the pH (“power of Hydrogen”). pH is important because it affects the ______at which chemical reactions occur, and must be _________ in order for the body to function properly.
- rate
- homeostatic
Acids are _______donors.
- proton
- Release H+ (a proton, or hydrogen ion) in solution
- HCl > H+ + Cl–
- HCl, HC2H3O2 (HAc), and H2CO3
Bases are proton ______.
- acceptors
- Take up H+
- Na+ + OH–
- OH– accepts an available proton (H+)
- OH– + H+ à H2O
- Bicarbonate ion from solution (HCO3–)
- NaOH and ammonia (NH3)
relative free [H+] of a solution is measured on the ___________.
- pH scale
As [OH-] decreases and [H+] increases…
- acidity increases
- pH lowers
As free [H+] decreases…..
- alkalinity increases
- [OH–] increases as [H+] decreases
- pH increases
Functional groups
Functional groups bond to carbon skeletons and are responsible for most of the chemical an physical properties of a particular organic compound
Name this group, why is it impotant biologically?

- Alcohol
- lipids and carbohydrates
Name this group, why is it important biologically?

- Aldehyde
- reduces sugars such as glucose; polysaccarides
- in and aldehyde molecule the carbon oxygen double covalent bond is on the end of the molecule.
Name this group, why is it important biologically?

- Methyl
- DNA; and energy metabolism
Name this group, why is it important biologically?

- Ketone
- metabolic intermediates
- the carbon oxygen double covalent bond is located inside the molecule instead of at the end
Name this group, why is it important biologically?

- Amino
- Proteins
- can also be written NH2
Name this group, why is it important biologically?

- Ester
- bacterial and eukaryotic plasma membranes
Name this group, why is it important biologically?

- Ether
- Archaeal plasma membranes
- the O, 2 Cs and the 4 Hs
Name this group, why is it iportant biologically?
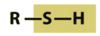
- Sulfahydrl
- energy metabolism and protein structure
- can also be SH
Name this group, why is it important biologically?

- Carboxyl
- organic acids, lipids and proteins
Name this group, why is it important biologically?

- Phosphate
- DNA; ATP
Identify the functional groups in an amino acid.
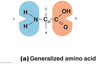
- Amino group
- Hydrogen
- Carboxyl group (acid group)
- Some form of functional group (replaceable)
*An amino acid can be made polar or nonpolar by different functional groups.
What do amino acids make?
proteins
What are the types of chemical reactions?
-
Dehydration synthesis
- Anabolic reaction; builds a dimer, how polymers are made.
- Endergonic reaction; uses up energy
- Monomers form covalent bonds when OH is removed from one monomer and H is removed from the other (at bonding site).
- Chemical water is removed to make a covalent bond. -
Hydrolysis
- Catabolic reaction; breaks down polymers into monomers.
- Exergonic reaction; releases energy
- The covalent bonds between monomers are broken apart by a water molecule; OH is added to one monomer and H is added to the other monomer.
to find out how many water molecules are made during a dehydration synthesis reaction what do you do?
- subtract 1 from the total amount of monomers in the polymer and the answer is the amount of water molecules made
- 100 monomers in a polymer —-> 100 - 1= 99
- 99 water molecules were made
how do you find out how many water molecules were used to slipt up a polymer into monomers?
- it takes 1 water molecule to spilt up a covalent bond between 2 monomers.
- OH bonds with one molecule and H bonds with the other- catabolic
- To break up 100 monomers you need 99 water molecules.
How do you tell different macromolecules apart?
- the elements that make up the molecule
- the ratio of thos elements
What are the 4 organic macro molecules?
- Carbohydrates
- Proteins
- Lipids
- Nucleic acids
These are all polymers
carbohydrates
Function: dietary energy; storage and plant structure
Monomer form: Monosaccharide
Examples: Monosaccharides: glucose, fructose,
Dissaccharides: lactose, sucrose
Polysaccharides: starch and cellulose
* carbohydrates are used in bacteria capsules allows bacteria to avoid immune cells*
Lipids
Function: longterm energy storage (for fats); hormones (for steriods)
Monomer: fat molecule made of glycerol (Hydrophilic) and fatty acid tail (Hydrophobic).
Examples: fats, oils, steriods.
*bacteria do not store lipids, they metabolize them to include it in their membrane*

Proteins
Functions: Enzymes, structure, storage, contraction, transport, etc.
Monomer: Amino acid
Examples: lactase (enzyme), hemoglobin
*proteins are the the only macromolecules can contain sulfur.*

Nucleic acids
Functions: information storage
Monomer: nucleotide
Examples: DNA and RNA

What molecules are being shown?

monosaccharides
what molecule is bing shown?

disaccaride
what molecules are being shown?

Polyschaccarides
what molecule is this?

- amino acid
- many amino acids bonded togther make a polymer (polypeptide chain or protein)
what molecule is this?

- fat molecule; glycerol and fatty acids
- many fat molecules bonded togther make lipids (polymer)
what molecule is this?

- nucleotide
- many nucleotides bonded togther form nucleic acids; DNA or RNA (polymers)
Dehydration synthesis and Hydrolysis work the same for all macromolecules?
True
bacteria do not store glucose as ___________.
glycogen
Triglyceride
1 glycerol plus 3 fatty acid chains; waters removed to make it
what does it mean if you see OH bonded on a molecule?
it can be dissolved in water.
why don’t lipids dissolve in water?
because they have no OH groups ; which means no polarity which means the fat molecules cannot hydrogen bond with water.
The formation of a fat molecule is accomplished through.
dehydration synthesis
The polar phosphate group in the head of a phospholipid molecule allows it to do what?
allows the outer and inner aspect of the cell to interact with watery eviornments in the ECF and cytoplasm.
what is the fluidity in the phospholipid bilayer, the property that makes the mosaic model possible.
the fluidity is caused by a bent unsaturated fatty acid chain; double bonds between 2 carbons, hydrogens are missing making it “unsaturated” with hydrogens.
____________ make up the bilayer in cell membranes.
- phospholipids
Bacteria do not tend to have ________ in their _____________.
- cholesterol
- membranes
Cholesterol is the basis for all __________in the body.
- steriods
in addition to fats lipids also include ___________ because it is __________.
- cholesterol
- non-polar
what type of molecule is this? How can you tell?

- A steriod hormone (testosterone)
- four interlocking hydrocarbon rings form a steroid.
what is the bond between 2 amino acids called?
peptide bond

- This is a poly peptide chain, a polymer of amino acids bonded togther by peptide bonds.
- R group
- Peptide bond
- Amino terminus (unbonded amine group)
- Carboxyl terminus (unbonded caboxyl group) (acid group)
proteins have a begining and ______.
end
peptide bonds between 2 amino acids make _______.
poly peptide chains (proteins)
Primary structure
- when amino acids form a poly peptide chain (amino acid sequence)
- caused by peptide bonds between amino acids
- the number of amino acids and the sequence of amino acids determine primary structure, shape and ability to function and interact with other molecules; shape determines function
- the special ribosomes of bacteria are the target of certain antibiotics. Protein synthesis disruption is the goal

Phospholipids: The “tails” are nonpolar and therefore do not _________________ Note that equal sharing of ______ among the carbons and hydrogens in the fatty acid tails creates the physical barrier of cell membranes, allowing cells to regulate what ______________.
- dissolve or interact with water
- electrons
- goes in and out of the cell
secondary structure
- the polypeptide chain forms alpha helices (spirals) or beta pleted sheets.
- hydrogen bonding between hydrogens and oxygens on amino acids cause spiraling and folding to occur.

what is the job of cholesterol in the phospholipid bilayer?
to control how fluid the membrane is
what determines the function of a protein?
it’s structure level
two peptides bonded togther
dipeptide
How many amino acids are there?
20
the R group of an amino acid does what?
The R group gives each amino acid unique qualities, such as polarity (allows it to hydrogen bond with other R groups in the protein), electrical charge (allows amino acid’s R group to have an ionic bond with other R groups in the protein)
Tertiary structure
- R group interaction cause further folding which results in tertiary folding.
- R group interactions:
- ionic bonds
- covalent bonds (disulfide bridges)
- hydrogen bonds
- hydrophobic interactions
- All proteins have a primary, secondary and tertiary structures.
- the secondary structured protein folds on itself again and forms a globular molecule held togther by the abovementioned bonds
- the inner most part of the globular molecule is hydrophobic and the outer most part is hydrophilic (hydrophobic packing)

Quaternary structure
- not all proteins have quaternary stucture
- two or more polypeptide chains (subunits) in tertiary structure come togther to create a protein with a quaternary structure (a functional protein)
- the same R group interactions in tertiary proteins cause quternary proteins to be formed
- ionic bonds
- covalent bonds
- Hydrogen bonds
- hydrophobic interactions
- Examples: Hemoglobin and collagen
- 4 subunits= tetromer 3 subunits= trimer
- 2 subunits= dimer

proteins can be come denatured what does this mean and how does it happen?
- protein loses its shape which effects its function
- this happens whenproteins are exposed to high temperatures or low pH
Enzymes
- proteins that catalyze chemical reactions in living things
- Enzymes make it easier for reactions to happen (less energy needed) —-> easier and faster to make and break chemical bonds.
how enzymes work
- enzymes are proteins so their shape is specific for their job
- subtrates bind to active sites on an enzyme forming a enzyme-substrate complex. (the enzyme’s shape changes
- the enzyme positions the substrates to were the atoms can interact with eachother
- when the bond is formed or broken (dehydration synthesis or hydrolysis) the substrates or products are released; the enzyme returns to it original shape. ready to aid more chemical reactions
DNA and RNA are __________.
nucleic acids
the monomer of a nucleic acid
nucleotide
what are the parts of a nucleotide?
- sugar-phosphate backbone
- Nitrogenous base (purine or pyrimidine)
- Adenine- pyrimidine
- Thymine (DNA)- purine
- Cytosine- purine
- Guanine-pyrimidine
- Uracil (RNA) - purine
Nucleotide monomers are bonded together in long chains by _________________ (what type of reaction breaks them apart?)
- dehydration synthesis reactions
- hydrolysis reactions
What is the difference between RNA and DNA?
- in DNA two polynucleotide (made of covalently bonded nucleotides) strands are hydrogen bonded and twisted into a helix shape.
- RNA is made up of only 1 polynucleotide strand.
- the sugars that make up the sugar-phosphate backbone differ-
- RNA: Ribose
- DNA: Deoxyribose
What are the 2 different types of nucleotides? what makes them different?
- purines- 2 rings
- pyrimidines- 1 ring
A (adenine) and G (guanine) are _________.
purines
T (thymine) and C (cytosine) and U (uracil) are _____________.
pyrimidines
ATP is a _________
- Nucleotide
- provides energy for cells
- made up of adenine, ribose and 3 phosphate groups
what makes ATP such a great energy source?
phosphate bonds are high in energy; when the bonds are broken down by hydrolysis it releases the energy pent up between electrons from oxygen molecules who have like charge repulsion and want to get away from eachother.
A….T what do the “…..” represent?
hydrogen bonds
Purines always H bond with ___________.
- pyrimidines
- the opposite is true
- A-T or U
- G-C
ATP _____________ proteins or reactants to allow __________, ________, provide _______These are all functions needed to maintain life.
- phoshorylates
- substances to be transported
- muscle cells to contract
- energy.
- waste removal
Base pair rules
- the specific way in which nucleotides H bond with eachother
- The rules of base pairing (or nucleotide pairing) are: A with T: the purine adenine (A) always pairswith the pyrimidine thymine (T) C with G: the pyrimidine cytosine (C) always pairs with the purine guanine (G)
Each strand of DNA has a ________ based on the numbering of the __________.
- direction
- carbon atoms
antiparallel
The two strands run in opposite directions
cells cannot use food molecules (monomers directly) they have to under go processing in the _________ to form ATP; a molecule that cells can use to do work.
- krebs cycle
ATP is actually a nucleotide, with three phosphate groups attached. Release of the third phosphate group provides energy for cellular work, and results in _______________. The chemical reaction is as follows: ATP —-> ADP + P
The reaction is also reversible; energy from food molecules is used to ______________ onto ADP to form ATP (the reaction is: ADP + P —–> ATP)
- ADP (Adenosine triphosphate) and inorganic phosphate
- Add phosphate back
As long as ATP is available, cells continue to perform their functions, and _________________.
- microorganism remains alive.
atomic number
number of protons in nucleus
Describe the structure of an atom and its relation to the physical properties of elements.
- nucleus made up of protons and neutrons
- electrons move around nucleus in electron shells
- same amt of protons and electrons
- the chemical properties of an atom rely mostly on the number of electrons in its outer shell.
atomic weight
the number of protons and neutrons in an atom added togther
electrons are arranged in _________ around atoms.
electron shells
electron configuration
- electron configuration is the distribution of electrons of an atom in atomic or molecular orbitals.
- shells of electrons layered outward away from the nucleus
- innermost shell carries 2, second shell carries 8 and the third shell (valence shell) carries 8
atoms can give up share or accept electrons in its valence shell in order to…
- become chemically stable
- Octet rule; each valence shell wants 8 e- ; so the closer a atom is to having 8 e- the more reactive it is.
How do atoms form molecules?
- through chemical bonding:
- ionic bonds
- covalent bonds
- hydrogen bonds
Why do molecules stay togther?
because the valence electrons of the combining atoms undergo chemical bonding
why do chemical bonds contain energy?
because energy is needed to form chemical bonds. chemical bonds hold potential energy.
ion
negatively (anion) or positively charged atom or group of atoms (by gaining or losing electrons)
ionic bond
an attracton between 2 atoms; one with a positive charge resulting from the lossof e- and one with a negative charge resulting from the gain of an e-
mole
the molecular weight of a substance in grams
molecular weight
the atomic weights of a molecule’s atoms added togther.
List several properties of water that are important to living systems.
- high boiling point, and low freezing point
- hydrogen bonds between water molecules affects density.
- universal solvent
- Water is a reactant or product in many chemical reactions. (important source of hydrogen and oxygen)
what pHdo most bacteria grow best in?
6.5-8.5 pH
the ratio of H to O atoms in a carbohydrate is
2:1
what is the general formula for carbohydrates
CH2O
simple lipids
- fats or triglycerides
- contain and alcohol called glycerol
- plus fatty acids
Complex lipids
- contain
- nitrogen
- phosphorus
- sulfur
- in addition to carbon, hydrogen and oxygen
- example of a complex lipid is a phospholipid. made up of a glycerol 2 fatty acids and in the place of the 3rd fatty acid a phosphate group.
- phospholipids build membranes
- phospholipids have polar and non polar regions
Cholesterol
- four fused carbon rings
- OH group attached to ring A makes it a sterol (cholesterol)
- separate fatty acid chains and prevent them from sticking togther in low temperatures; prevents plasma membrane from becomming rigid.
Proteins
- contain
- carbon
- hydrogen
- oxygen
- nitrogen and sometimes sulfur
- responsible for many different structure and function roles in a cell.
- Enzymes- speed up chem reactions in cells
- Transporter proteins- on surface of plasmamembran transport substances in and out of the membrane.
- bacteriocins- poisins bacteria make to kill other bacteria
- exotoxins- produced by some microbes
- hormones
- contraction and movement
- antibodies
peptide
4-9 amino acids
polypeptide
10-2000 or more amino acids
what does a nucleotide consist of?
- nitrogenous base (purine or pyrimidine)
- Pentose sugar
- phosphate group
How do DNA and RNA differ?
- RNA is single stranded
- contains ribose instead of deoxyribose
- contains uracil instead of thymine
- RNA aids in protein synthesis and DNA is responsible for storing determining genetic information.
What is ATP made of?
- adinine
- ribose
- 3 phosphate groups bonded to eachother and the ribose molecule


