Chemical Reactions Flashcards
1
Q
Chromate
A
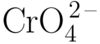
2
Q
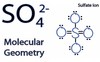
Sulfate
A
3
Q
carbonate
A

4
Q
Hydroxide
A

5
Q
Chlorate
A

6
Q
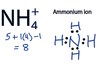
ammonium
A
7
Q
acetate
A

8
Q
nitrate
A

9
Q
phosphate
A

10
Q
dichromate
A

11
Q
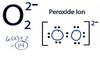
peroxide
A
12
Q
ammonia
A

13
Q
cyanide
A

14
Q
sulfuric acid
A

15
Q
nitric acid
A

16
Q
permanganate
A

17
Q
hydrochloric acid
A

18
Q
acetic acid
A
19
Q
dissociation
A
The seperation of ions that occurs when an ionic compound dissolves
20
Q
spectator ions
A
Ions that do not participate directly in the reaction.
21
Q
net ionic equation
A
Equation that includes only the compoments directly involved in the reation.
22
Q
synthesis
A
A + X = AX
Two or more substances combine to form a new compound.
23
Q
decomposition
A
AX = A + X
A more complex compound reacts to form two or more simple compounds.
24
Q
single displacement
A
A + BX = B + AX
Y + BX = X + BY
One element replaces a similar element in a compound.
25
double displacement
AX + BY = AY + BX
The ions of two compounds trade places in an aqueous solution to form two new compounds, one of which is usually a solic, liquid, or gas.
26
combustion
CxHx + O2 (g) = flame and different kinds of products
A substance combines with oxygen producing hear so rapidly that a flame results.
27
seven diatomic molecules
(7, 7, 7)
nitrogen, bromine, oxygen, hydrogen, fluorine, chlorine, iodine
28
molecular equation
Shows all reactants and products of a reaction, but does not give a very clear picture of what actually occurs.
29
strong electrolyte
A substance that completely breaks apart into ions when dissolved in water.
30
molarity
M/L
31
solvent + solute =
solution
32
Two synthesis reactions that happened when the magnesium was heated
combined with O2
combined with N2
33
How did you prove that the nitrogen gas had reacted with the magnesium?
It would make Mg3N2 which reacts with water to form NH3 (smell of ammonia)
34
Write an equation for what happened to the sugar.
C6H12O6 ---\> 6C + ^ 6H2O
Above the arrow is H2SO4
35
Write the balanced equation for the double displacement reaction you did with lead.
Pb(NO3)2 + 2KI
switched and balanced
36
Hydrogen takes what form as a diatomic molecule?
gas
37
Nitrogen takes what form as a diatomic molecule?
gas
38
Oxygen takes what form as a diatomic molecule?
gas
39
Fluorine takes what form as a diatomic molecule?
gas
40
Chlorine takes what form as a diatomic molecule?
gas
41
Bromine takes what form as a diatomic molecule?
liquid
42
Iodine takes what form as a diatomic molecule?
solid
43


