Chapter 4: Energy, Enzymes, and Cellular Metabolism Flashcards
What is bioenergetics?
The flow of energy in living systems such as the conversion of light energy into glucose by plants.
Also obeys Laws of thermodynamics:
What forms of work can energy carry out?
Chemical work
- Making and breaking of chemical bonds
Transport work
- Moving ions, molecules
- Concentration gradients
Mechanical work
- Moving organelles, changing cell shape, beating flagella and cilia
- Contracting muscles
Explain Activation energy.
Energy required for the reactants to react.
Most molecules lack energy for a reaction.
- Heat increases the likelihood or rate of reaction
- But heat has some negative effects on cells
- Catalysts help reactions occur at lower temperatures

What are Enzymes and what do they do?
A class of proteins that serve as catalysts Chemicals that:
- Increase the rate of a reaction
- Are not changed by the reaction
- Do not change nature of reaction

Describe the mechanisms of enzymes.
- The function of an enzyme is determined by its structure
- Each enzyme has a characteristic 3D shape or conformation, with pockets that serve as active sites in the enzyme
- The reactants are called substrates, and they fit into this specific active site like a key to a lock

How do Substrates interact with enzymes?
When a substrate binds to the active site of an enzyme, it forms temporary bonds, weakening the original bonds of the substrate
New bonds are formed between substrates as they are brought close together by the enzyme
- Bonding of enzyme to substrates forms a temporary enzyme-substrate complex
- This breaks to yield the products of the reaction
- The amount of enzyme in a sample determines the rate of product produced

How are names for enzymes created?
- The first enzymes were given arbitrary names
- An international committee decided all enzyme names end with the suffix –ase
- Names apply to the function of the enzyme
- Phosphatases remove phosphate groups
- Synthetases catalyze dehydration synthesis
- Enzymes are often produced in an inactive form and activated when needed such as
- Pepsinogen -pepsin
How is enzyme acticity measured and what influences it?
- Measured by the rate at which substrate is converted to product
- Influenced by:
- Temperature
- pH
- Concentration
- Cofactors and coenzymes
- Stimulatory or inhibitory substances
What are the effects of temperature on enzymes?
Temperature will increase the rate of reaction to a point and then the enzyme will be denatured

What are the effects of pH on enzymes?
Enzymes exhibit peak activity within a narrow pH range = pH optimum
- Due to changes in enzyme conformation
- Optimum pH reflects environment enzyme is found

What are coenzymes?
- Most enzymes need additional small molecules to aid in a reaction
- Coenzymes are organic molecules derived from water-soluble vitamins
Ex: NAD from vitamin niacin (B3) - Coenzymes transport hydrogen atoms and other small molecules between enzymes
- Cofactors are metal ions such as: Ca2+ Mg2+ Mn2+ Cu2+ Zn2+
- Cofactors help form the active site or enzyme-substrate binding

Explain the effects of substrate concentration on reaction rate.
As the substrate concentration increases, so will the rate of reaction until the enzyme is saturated = every enzyme is busy

What are metabolic pathways?
- Metabolic pathways are series of chemical reactions occurring within a cell. In each pathway, a principal chemical is modified by a series of chemical reactions. Enzymes catalyze these reactions, and often require dietary minerals, vitamins, and other cofactors in order to function properly.
- Most reactions are linked together in a chain called a metabolic pathway
- Begin with an initial substrate
- End with a final product
- Many enzymatic steps along the way

How do cells regulate their metabolic pathways?
- Controlling enzyme concentrations
- Chemicals that change reaction rates
- Using different enzymes to catalyze reversible reactions
- Compartmentalizing enzymes within organelles
- Maintaining optimum ratio of ATP to ADP
What are reversible reactions?
- A reversible reaction is a chemical reaction where the reactants form products that, in turn, react together to give the reactants back.
- Sometimes a single enzyme can drive a reaction in two directions, depending on the concentration of substrate vs product
- When one gets higher, the reaction reverses
Example: carbonic anhydrase
H2O + CO2 ↔ H2CO3 ↔ HCO3- + H+

What are endergonic reactions?
- Chemical reactions that require input of energy
- Products contain more free energy than reactants
Plants need the energy from light to turn carbon dioxide and water into glucose

What are exergonic reactions?
- Chemical reactions that produce energy
- Products have less free energy than reactants
Breaking glucose down into carbon dioxide and water produces energy
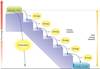
What are coupled reactions and how do they work?
- A coupled biochemical reaction is one where the free energy of a thermodynamically favourable reaction (such as the hydrolysis of ATP) is used to ‘drive’ a thermodynamically unfavourable one, by coupling or ‘mechanistically joining’ the two reactions.
- To put it another way, two (or more) reactions may be combined by an enzyme (for example) such that a spontaneous reaction may be made ‘drive’ an unspontaneous one.
- So, Energy from the exergonic break down of food drives endergonic reactions in our bodies
- Energy must be converted into a usable form:
The ATP molecule stores energy in its bonds
What is Oxidation, Reduction, and Redox?
- Reduction: atom or molecule gains electrons
- Oxidation: atom or molecule loses electrons
- Redox: for one molecule to lose an electron, it has to give it to another molecule
- Molecules can be both oxidizers and reducers in a chain reaction where e- are passed along
Are free electrons or hydrogen atoms passed along during oxidation/reduction?
Usually, free electrons are not passed along, but hydrogen atoms carrying the electrons are
- A molecule that loses hydrogen is oxidized
- A molecule that gains hydrogen is reduced
What are the oxidized/reduced states of NAD and FAD?
- Each NAD+ can accept 2 electrons and bind to 1 proton being reduced to NADH
- Each FAD can accept 2 electrons and bind to 2 protons being reduced to FADH2

What are the two reactions of metabolism?
All reactions in the body can be divided into:
- Anabolism: requires input of energy to synthesize large molecules from smaller ones
- Catabolism: releases energy stored in bonds by breaking down large molecules into smaller ones
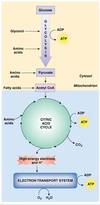
How does the catabolism of nutrients work?
- Catabolic reactions that break down nutrients serve as energy sources for synthesis of ATP
Glucose, fatty acids, and amino acids - Complete breakdown of glucose requires
Oxygen as the final electron acceptor, aerobic
Many steps such as first ones are anaerobic

Give a brief overview of energy metabolism