Chapter 26 - Carbonyls and carboxylic acids Flashcards
What is the carbonyl group?
C=O
What does oxidation of an aldehyde form?
Carboxylic acid
What are the reagents and conditions of oxidation of an aldehyde?
- Reflux
- aldehyde + pottasium dichromate (VI) ions, K2Cr2O7 + H2SO4

Do ketones undergo oxidation?
No
What effect does C=O bond have on the reactivity of carbonyls compared to the C=C bond of alkenes?
C=O bond is polar, therefore aldehydes and ketones can react with some nucleophiles. Nucleophiles attacks the slightly positive carbon atom resulting in addition across the C=O bond (nucleophillic addition)
Non polar C=C bond in alkenes recats with electrophiles by electrophillic substitution.

How is a reducing agen represented in an equation?
[H]
What is the reducing agent in reduction of ketones and aldehydes to alcohols
(aldehyde/ketone warmed with) NaBH4 in aqueous solution
What do aldehydes reduce to?
Primary alcohols
Write the equation for the reduction of butanal

Write the equation for reduction of propanone

What happens when HCN is reacted with aldehydes or ketones?
Adds across C=O bond, forming a hydroxynitrile (contains -OH and C=-N funstional groups)

How is the HCN provided in this reaction? Why can’t HCN just be added?
NaCN and H2SO4 are used to provide the hydrogen cyanide.
HCN is a colourless, extremely poisonous liquid that boils slightly above room temp, so it can’t be used safely in an open lab
What kind of reactions are the reduction of aldehydes/ketones and reaction with HCN?
nucleophillic addition
Write the mechanism for the reduction of aldehydes/ketones with NaBH4
NaBH4 can be considered as containing the hydride ion, :H-, which acts as a nucleophile
- ) The lone pair of electrons from the hydride ion, :H- is attracted and donated to the delta+ carbon atom in the aldehyde or ketone C=O double bond
- ) A dative covalent bond is formed between the hydride ion and the carbon atom of the C=O double bond
- ) The π-bond in the C=O double bond breaks by heterolytic fission, forming a negatively charged intermediate
- ) The oxygen atom of the intermediate donates a lone pair of electrons to a hydrogen atom in a water molecule. The intermediate has then been pronated to form an alcohol.

Write the mechanism for the reaction of aldehydes/ketones with HCN (NaCN/H+)
cyanide ion, :CN- attacks the electron-deficient carbon atom in the aldehyde or ketone.
- ) The lone pair of electrons from the cyanide ion, :CN- is attracted and donated to the delta+ carbon atom in the aldehyde or ketone C=O double bond. A dative covalent bond forms.
- ) The π-bond in the C=O double bond breaks by heterolytic fission, forming a negatively charged intermediate.
- ) The intermediate is pronated by donating a lone pair of electrons to a hydrogen ion, to form the product
- ) Product is a hydroxynitrile.

What is used to detect a carbonyl fuctional group?
2,4-dinitrophenylhydrazine (2,4-DNP) also known as Brady’s reagent
yellow or orange precipitate of 2,4-dinitrophenylhydrazone produced in presence of carbonyl group
Describe the procedure of testing for the carbonyl group using 2,4-DNP (brady’s reagent)
- ) Add 5cm3 of 2,4-DNP to a clean test tube. This is in excess
- ) Using a dropping pipette, add 3 drops of the unknown compound. Leave to stand.
- ) If no crystals form, add a few drops of sulphuric acid.
- ) A yellow/orange precipitate indicates the presence of an aldehyde/ketone.
Which reagent ca distinguish between an aldehyde and ketone?
Tollens’ reagent
(a solution of silver nitrate in aqueous ammonia)
in presence of an aldehyde group, a silver mirror is produced.
Describe the procedure of testing for an aldehyde group using tollens’ reagent
Tollens’ reagent made up by:
1.) In a clean test tube, add 3cm depth of aqueous silver nitrate, AgNO3(aq).
- ) Add aqueous sodium hydroxide to the silver nitrate until a brown precipitate of silver oxide, Ag2O is formed.
- ) Add dilute ammonia solution until the brown precipitate just dissolves to form a clear colourless solution - tollens’ reagent
carrying out test for an aldehyde:
- ) Pour 2cm depth of the unknown solution into a clean test tube
- ) Add an equal volume of freshly prepared Tollens’ reagent
- ) Leave the test tube to stand in a beaker of warm water at about 50ºC for about 10 to 15 minutes and then observe whether any silver mirror is formed. If so then the unknown solution contains an aldehyde.If the unknown solution is a ketone then no reaction is observed
Give the oxidation and reduction reactions that take place in a reaction between an aldehyde and silver ions
reduction:
Ag+(aq) + e- → Ag(s)
oxidation:
R-CHO + [O] → R-COOH
silver mirroe formed from reduction of silver
Describe the procedure of identifying an aldehyde or ketone by melting point
2,4-dinitrophenylhydrazone precipitate formed in the 2,4-DNP test can be analysed by:
- ) The impure yellow/orange solid is filtered to seperate the solid precipitate from the solution.
- ) The solid is then recrystallised to produce a pure sample of crystals
- ) Melting point of the purified 2,4-dinitrophenylhydrazone is measured an recorded.
- ) melting point compared to a database to identify original carbonyl compound
Why are carboxylic acids (with up to 4 carbons) soluble in water?
C=O and O-H bonds in carboxylic acids are polar allowing hydrogen bonds to form with water molecules
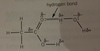
Why are carboxylic acids only soluble with up to 4 carbon atoms?
As the number of atoms increases, the solubility decreases as the non-polar carbon chain has a greater effect on the overall polarity of the molecule.
Are carboxylic acids strong or weak acids?
- Weak acids
when dissolved in water, carnoxylic acids partially dissociate:
HCOOH(aq) ⇔ H+(aq) + HCOO-(aq)












