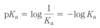Chapter 2: Water Flashcards
(92 cards)
_____ ______ Gives Water Its Unusual Properties
Hydrogen Bonding
Lecture
because of the unequal electron sharing between each H and O in water, each hydrogen atom bears a ____ _____ charge, and the oxygen atom bears a ______ ______ charge equal in magnitude to the _____ of the _____ ______ _____. As a result, there is an _____ ______ between the oxygen atom of one water molecule and the hydrogen of another called a _____ ______
- partial positive charge (δ+)
- partial negative charge
- sum
- two partial positives (2δ−)
- electrostatic attraction
- hydrogen bond
Lecture
The nearly tetrahedral arrangement of the orbitals about the oxygen atom allows each water molecule to form hydrogen bonds with as many as ____ neighboring water molecules
four
Lecture
When ice melts or water evaporates, _____ is taken up by the ____
- heat
- system
Lecture
Gibs free energy focuses on the _____
system
Lecture
Gibs free energy formula
ΔG = ΔH - TΔS
ΔG: gibbs free energy
ΔH: enthalpy
T: temp in Kelvin
ΔS: entropy
Lecture
Gibs free energy summary
- ΔG is proportional to the negative of ΔSuniv
- ΔG < 0 = spontaneous process
- ΔG > 0 = non-spontaneous process
Lecture
Gibs free energy formula when
ΔH < 0 and ΔS > 0
ΔH (enthalpy) < 0: heat is emitted ↑ entropy
ΔS (entropy) > 0: entropy ↑
- both are increasing entropy so universe ↑ entropy → spontaneous
- both enthalpy and entropy favor the reaction

Lecture
Gibs free energy formula when
ΔH < 0 and ΔS < 0
enthalpy < 0: heat is emitted ↑ entropy
entropy < 0: entropy ↓
temp: low ↑ entropy
@ low temps, heat emitted = ↑ entropy
so universe ↑ entropy → spontaneous
@ hi temps, heat dispersed to warmer sorroundings makes change in ↑ of entropy small, insignificant
- enthalpy favors the reaction
- entropy opposes
- “enthalpy driven” reaction

Lecture
Reactions will tend to go from _____ to _____ if they give _____ _____ OR if they involve a _____ of order (increase in _____). The actual direction depends on the balance of favorable and unfavorable contributions to both the enthalpy and entropy.
- left
- right
- off heat
- loss
- randomness
Lecture
Gibs free energy formula when
ΔH > 0 and ΔS > 0
enthalpy > 0: heat is absorbed ↓ entropy
entropy > 0: entropy ↑
temp: high ↑ entropy
@ hi temps, heat emitted = ↑ entropy
so universe ↑ entropy → spontaneous
@ absorption of heat (by ΔH) from surrounding has less effect on entropy as temp increases
- unusual, entropy driven
- absorbs heat but is favored by the large entropy increase resulting from the formation of gaseous products
Lecture
Hydrogen bonds form between an ______ atom (the hydrogen _____) and a hydrogen atom covalently bonded to another ______ atom (hydrogen _____). common hydrogen acceptors are
- electronegative
- acceptor
- electronegative
- donor
- oxygen, nitrogen, flourine
Hydrogen atoms covalently bonded to carbon atoms ______ participate in hydrogen bonding, because carbon is ______ more electronegative than hydrogen and thus the C—H bond is only very _____ _____
- don’t
- slightly
- weakly polar
Lecture
Hydrogen bonds are strongest when the bonded molecules are oriented to maximize electrostatic interaction. That is when the H & the two atoms that share it are in a _____ _____
straight line

Lecture
Amphipathic compounds
compounds that contain regions that are polar (or charged) and
regions that are nonpolar
Lecture
When an amphipathic compound is mixed with water, the ____ (_____) region interacts favorably with the water and tends to dissolve, but the _____ (______) region tends to avoid contact with the water
- polar
- hydrophilic
- nonpolar
- hydrophobic
Lecture
hydrophobic effect
in amphipathic compounds the nonpolar regions of the molecules cluster together to present the smallest hydrophobic area to the aqueous solvent, and the polar regions are arranged to maximize their interaction with the solvent
Lecture
micelles
- lipid molecules that arrange themselves in a spherical form in aqueous solutions
- form in response to the amphipathic nature of fatty acids, meaning that they contain both hydrophilic regions (polar head groups) as well as hydrophobic regions (the long hydrophobic chain)
Lecture
The _____ ______ on interactions among lipids, and between lipids and proteins, is the most important determinant of structure in biological membranes
hydrophobic effect
Lecture
Water Forms Hydrogen Bonds with _____ Solutes
polar
Lecture
Entropy Increases as _____ Substances Dissolve
Crystalline (ie. NaCl)
_____ Gases Are Poorly Soluble in Water
Nonpolar
van der Waals Interactions Are Weak _____ Attractions
Interatomic
van der Waals interactions aka London forces
- When two uncharged atoms are very close together
- their surrounding electron clouds influence each other
- variations in the positions of electrons create a transient electric dipole in one, and a opposite in the other
- The two dipoles weakly attract each other, bringing the two nuclei closer
- As the two nuclei draw closer together, their electron clouds begin to repel each other
- where net attraction is maximal, the nuclei are said to be in van der Waals contact
- Each atom has a their own van der Waals radius, a measure of how close that atom will allow another to approach