Bone cell biology Flashcards
What are the functions of bone?
- infrastructure
- bone marrow
- reservoir for Ca and phosphate
- specialized connective tissue; composed of cells and matrix (bone is calcified ECM)
What regulates the calcium stored in bone?
- Parathyroid Hormone (PTH) stimulates bone resorption and calcium release, via PTH-induced osteoclasts
- Calcitonin (from the thyroid) antagonizes this process by inhibiting osteoclasts
What makes up the bone matrix?
- inorganic part (70%)
- calcium + phosporus= hydroxyapatite
- hydroxyapatite makes bone hard
- stores 99% of the body’s Ca
- organic part (30%); osteoid
- type I collagen (stains eosinic/red)
- proteoglycans (less than in cartilage)
-
glycoproteins (promote calcification/hydroxyapatite)
- e.g. osteopontin, osteocalcin, which is bone-specific, and sialoprotein
Compare the mineral/water content, collagen type, and neuronal/vascular structure of bone versus hyaline cartilage

What makes an osteoblast?
Osteoblasts are specialized fibroblasts that differentiate from mesenchymal stem cells (MSCs) due to growth factors including bone morphogenetic proteins (BMPs)
What is the function of osteoblasts?
- Groups of osteoblasts make osteoid (the bone matrix; type I collagen and glycoproteins)
- Osteoblasts in the periosteal layer mediate increased width (appositional growth; can be monitored by tetracyclin injections)
What is Runx2?
A transcription factor considered as the bone ‘master gene’, without which bone cannot form
(Runx2 and osteocalcin are osteoblast specific)
What is osteomalacia?
A condition in which calcification is impaired
What is osteitis fibrosa cystica?
A condition where osteoclasts destroy the bone matrix as it is made
What are osteocytes?
**differentiated osteoblasts
- occupy lacunae (similar to cartilage cells), which lie between layers of bone matrix termed lamellae
- ONE osteocyte per lacuna
- comprise 90% of all bone cells
- live a long time (half life ~25 years!)
- aging is accompanied by significant osteocyte loss
What is the function of osteocytes?
- cytoplasmic processes (dendrites) penetrate the bony matrix via canaliculi, where they bind to all types of bone cells via gap junctions
- function as endocrine cells
- regulate mechano-sensation (cell to cell signaling) which regulates bone remodeling
- modulate the opposing activities of osteoblasts and osteoclasts
- secrete sclerostin (inhibits Wnt signaling in osteoblasts, thereby stopping growth)

What are osteoclasts?
- large (~50 μm) multinuclear cells (15+ nuclei), formed in the bone marrow by the fusion of monocyte-like cells
- occupy Howship’s lacunae (partly hollowed-out regions of matrix along the endosteal surface)
- clamp down onto the matrix via a r_uffled cellular border,_ which forms a microenvironment in which the bone matrix is resorbed

What is the function of osteoclasts?
- destroy bone matrix for remodeling
- Activated osteoclasts release protons -> lowers pH
- > dissolves calcium phosphate (hydroxyapatite) -> lysosomes release cathepsin-K (enzyme) into microenvironment
- Activated by cytokines such as parathyroid hormone (PTH), and are inhibited by the thyroid hormone calcitonin
**osteoclasts overactive in osteoporosis
What is Paget’s disease?
- abnormal osteoclasts cause an abnormally high rate of remodeling
- results in an over-abundance of relatively weak immature bone termed primary or woven bone
- afflicts adults over 40 years old

What are the two types of bone?
- compact (cortical)
- areas of dense bone without cavities
- ~80% of long bone
- spongy (cancellous, trabecular)
- areas in which bone is highly trabeculated and cavitated
- ~20% of long bone

Contrast long and flat bones
- Long Bones
- diaphysis (shaft) consisting mostly of compact bone, with spongy bone lining marrow
- epiphyses “caps” of compact bone surrounding spongy bone
- Flat Bones
- calvarial bones of the skull
- two plates of compact bone surround the diplöe (an area of spongy bone)
What are the bone linings and where are the cell types located within them?
**periosteum (outer connective tissue layer) and endosteum (inner connective tissue layer)
- osteoblasts= periosteum (small amt in endosteum)
- osteocytes= within lacunae of the bony matrix between the periosteum and endosteum
- osteoclasts= endosteum attached to the bony matrix

What is an osteon?
- Osteon= a cylindrical bone subunit
- Each osteon has concentric lamella of bone
- lamella have lacunae between them that harbor osteocytes
- lacunae are connected via canaliculi
- collagen type I fibers in alternating lamellae are arranged in anti-helical fashion (increases bone strength despite its light weight)
- The innermost lamella surrounds the Haversian canal
- Haversian canals are connected perpendicularly via Volkmann’s canals
- both Haversian and Volkmann contain blood vessels, nerves, and lymph vessels

Contrast primary and secondary bone
- primary= “woven”
- less organized
- observed in developing or regenerating bone.
- secondary= “lamellar”/mature
- found in mature adult bone
What are the two ways bone can develop?
- Intramembranous
- osteoblasts deposit osteoid onto a loose framework of reticular connective tissue
- FLAT bones
- Endochondral
- osteoblasts deposit osteoid onto hyaline cartilage
- LONG bones
Describe how flat bones form
**intramembranous ossification (e.g. how fontanelles in neonates are filled-in with bone)
- bone forms in islands termed ossification centers
- groups of mesenchymal stem cells (MSCs) condense and differentiate into osteoblasts within a loose framework of reticular connective tissue
- bone matrix is secreted and calcified upon framework
- ultimately osteocytes are incorporated into lacunae
Describe how long bones form
**endochondral ossification
- osteoblasts deposit bone matrix on pre-existing template of hyaline cartilage
- begins in the diaphysis (in the primary ossification center)
- hypertrophy of the cartilage cells
- osteoblast-mediated bone production on the matrix of calcified cartilage (blasts secrete osteoid -> ossification)
- At the epiphyses, mostly the same process but some hyaline cartilage is retained as…
- articular cartilage
- epiphyseal plate cartilage (allows bone length growth)
“How do long bones get long”?
Estrogen/testosterone induce the
pituitary to express growth hormone, which in turn induces
the liver to make insulin-like growth factor-1 (IGF-1)
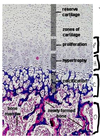
What are the 4 zones of long bone?
- Zone of Cartilage Proliferation (activated by IGF-I)
- Zone of Hypertrophy **susceptible to fracture (20% of fractures in children) because of large lacunae
- Zone of Calcification; chondrocyte proliferation is inhibited, collagen type X is synthesized, and hydroxyapatite formation begins **this region to be strongly basophilic
- Zone of Ossification; osteoblasts make collagen type I, **this area is strongly eosinophilic